Connor Perrett
President Trump defended his golf outings as his "exercise" in a Sunday morning tweet. Getty
President Donald Trump defended his golfing trips in a Sunday morning tweet.
"My 'exercise' is playing, almost never during the week, a quick round of golf," the president said, adding "Obama played more and much longer rounds, no problem."
President Donald Trump defended his golfing trips in a Sunday morning tweet.
"My 'exercise' is playing, almost never during the week, a quick round of golf," the president said, adding "Obama played more and much longer rounds, no problem."
By the end of May, Trump had spent some 266 days of his first term at one of his golf clubs, outpacing former President Barack Obama, who had golfed 98 times by the same point in time during his time in office, according to a CNN analysis.
Trump negatively tweeted about Obama's golf outings more than two dozen times while Obama was president.
According to a CNN tally, Trump had spent 266 days at one of his golf clubs by the end of May, while Obama had played 98 rounds by that point in his presidency.

President Donald Trump on Sunday defended his frequent golf outings in a tweet Sunday despite counts that say he has outpaced former President Barack Obama's golf rounds.
"I know many in business and politics that work out endlessly, in some cases to a point of exhaustion. It is their number one passion in life, but nobody complains," Trump wrote. "My 'exercise' is playing, almost never during the week, a quick round of golf. Obama played more and much longer rounds, no problem."
His tweet comes just two weeks after his aides reportedly struggled to reach him while he was golfing. On June 28, the president shared a video of a group of his supporters clashing with anti-Trump protesters in Florida. In the video, an apparent supporter begins to chant "white power." Trump shared the video, tweeting the clip showed "great people."
The video remained on the president's Twitter feed for three hours before it was taken down. A campaign spokesperson later said later that the president "did not hear" the phrase, which was uttered early in the video, before he shared the video.
According to NBC News, White House aides reportedly struggled to contact the president to get him to remove the video but were unable to reach him for about three while he was golfing at the Trump National Golf Club in Sterling, Virginia and had put his phone down.
White House aides reportedly struggled to contact the president to remove a video he retweeted where a supporter chanted "white power." Donald Trump/Twitter"When I play, Fake News CNN, and others, park themselves anywhere they can to get a picture, then scream 'President Trump is playing golf,'" the president continued in a second tweet on Sunday. "Actually, I play VERY fast, get a lot of work done on the golf course, and also get a 'tiny' bit of exercise. Not bad!"
Trump has golfed more times than Obama had by this point in their first terms
While Trump claimed that he has golfed fewer times than his predecessor, that's not accurate, according to a CNN fact check from May. Trump has spent 266 days at his golf clubs at the end of May while Obama had played 98 rounds by that point in his presidency.
The president had paused his frequent trips to the golf course when the COVID-19 pandemic first reached the US but has resumed his outings since the end of May. In May, Trump also defended his golfing as the COVID-19 US death toll neared 100,000. Now, at least 134,817 people have died from the coronavirus in the US, according to a tally by Johns Hopkins University.

According to Trump Golf Count, a website that tracks the president's golf outings, Obama played 306 rounds of golf during his eight years in office — just 45 more than the 261 it estimates Trump has already played during his first term. At his current rate, Trump would golf 596 rounds by the end of a hypothetical second term, Trump Golf Count projected.
Trump has regularly criticized then-President Barack Obama for golfing while he was president. According to SBNation, the then-reality show host tweeted critiques about Obama playing golf during his time as president at least 27 times.
"We pay for Obama's travel so he can fundraise millions so Democrats can run on lies," Trump tweeted in October 2014. "Then we pay for his golf."
—Donald J. Trump (@realDonaldTrump) November 18, 2013
"Can you believe that, with all of the problems and difficulties facing the US, President Obama spent the day playing golf," Trump said in another October 2014 tweet.
AND HIS EXCUSES GET EVEN BETTER AS THE DAY GOES ON

 Slate Magazine
Slate Magazine
Trump Insists Obama Played More Golf as President. The Numbers Say Otherwise.
While Trump claimed that he has golfed fewer times than his predecessor, that's not accurate, according to a CNN fact check from May. Trump has spent 266 days at his golf clubs at the end of May while Obama had played 98 rounds by that point in his presidency.
The president had paused his frequent trips to the golf course when the COVID-19 pandemic first reached the US but has resumed his outings since the end of May. In May, Trump also defended his golfing as the COVID-19 US death toll neared 100,000. Now, at least 134,817 people have died from the coronavirus in the US, according to a tally by Johns Hopkins University.

According to Trump Golf Count, a website that tracks the president's golf outings, Obama played 306 rounds of golf during his eight years in office — just 45 more than the 261 it estimates Trump has already played during his first term. At his current rate, Trump would golf 596 rounds by the end of a hypothetical second term, Trump Golf Count projected.
Trump has regularly criticized then-President Barack Obama for golfing while he was president. According to SBNation, the then-reality show host tweeted critiques about Obama playing golf during his time as president at least 27 times.
"We pay for Obama's travel so he can fundraise millions so Democrats can run on lies," Trump tweeted in October 2014. "Then we pay for his golf."
—Donald J. Trump (@realDonaldTrump) November 18, 2013
"Can you believe that, with all of the problems and difficulties facing the US, President Obama spent the day playing golf," Trump said in another October 2014 tweet.
AND HIS EXCUSES GET EVEN BETTER AS THE DAY GOES ON
Trump Insists Obama Played More Golf as President. The Numbers Say Otherwise.
Numbers vary slightly. Mark Knoller of CBS News, for example, specifies that Trump has made 193 visits to golf courses over at least part of 258 ...

 New York Post
New York Post
President Trump defends golf as 'exercise,' says he gets work done on the course
“Obama played more and much longer rounds, no problem. ... “Actually, I play VERY fast, get a lot of work done on the golf course, ... According to Trump Golf Count, Trump has visited one of his golf courses 261 times since ...

 FOX 29 News Philadelphia
FOX 29 News Philadelphia
Trump defends trips to golf courses, says it's his 'exercise' and he plays 'very fast'
President Trump defends golf as 'exercise,' says he gets work done on the course
“Obama played more and much longer rounds, no problem. ... “Actually, I play VERY fast, get a lot of work done on the golf course, ... According to Trump Golf Count, Trump has visited one of his golf courses 261 times since ...
Trump defends trips to golf courses, says it's his 'exercise' and he plays 'very fast'
During his 2016 presidential campaign, then-candidate Trump attacked President Barack Obama for reports of the latter's visits to the links – ...
AND THE REASON FOR THIS LITTLE TWEET STORM,
DISTRACTION OF COURSE FROM THE BAD NEWS OF THE DAY
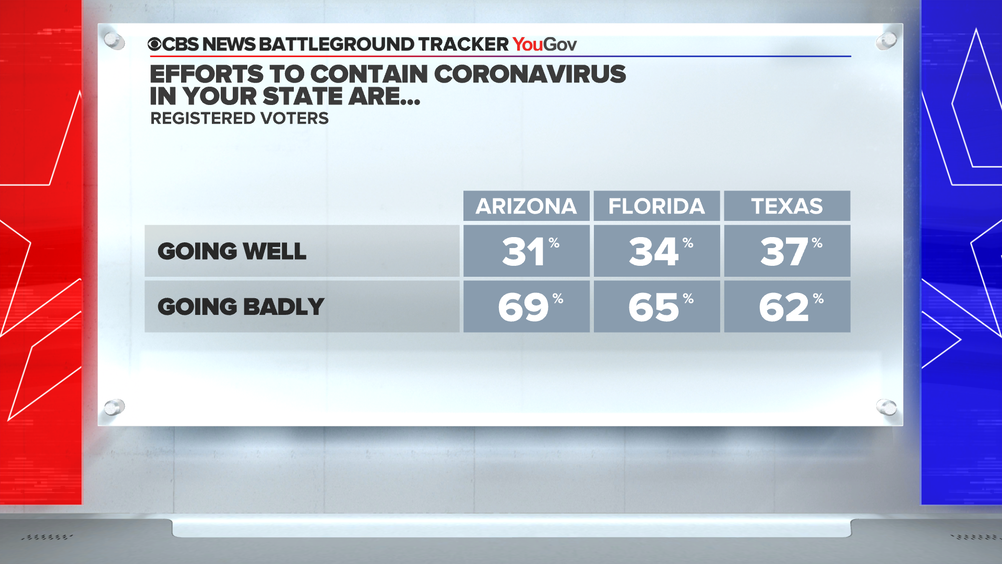
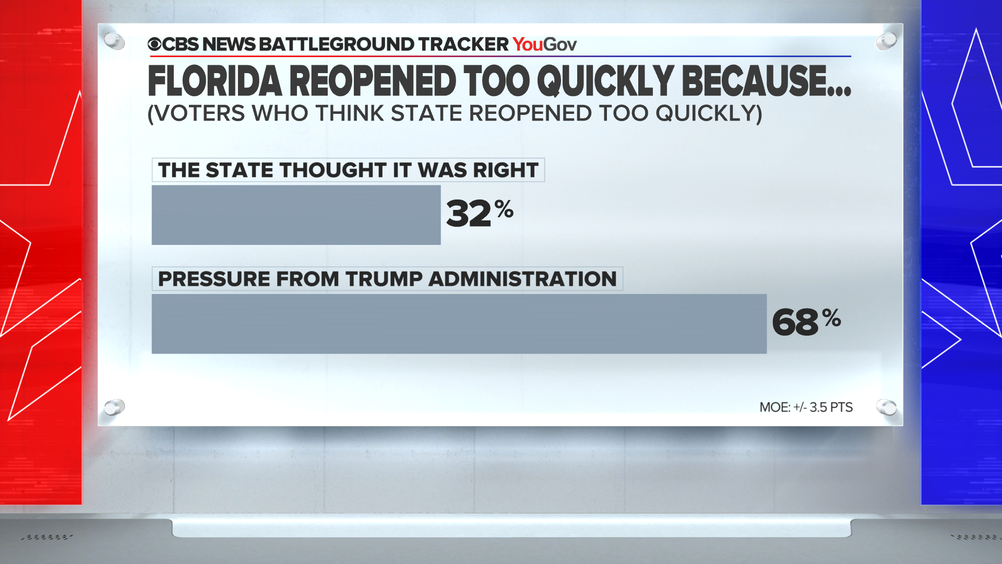
Virus outbreak reshapes presidential race in Sun Belt — CBS News Battleground Tracker poll
https://www.cbsnews.com/news/covid-presidential-race-sun-belt-opinion-poll-cbs-news-battleground-tracker/