Sophie underwent a cardiac ablation procedure in a Perelman School of Medicine translational research lab to treat her arrhythmia—the first time a dog with her diagnosis received such a treatment. Veterinary cardiologist Anna Gelzer says of the collaboration, “It’s the best of both worlds.”
 Cardiology resident Alexandra Crooks and cardiologist Anna Gelzer of the School of Veterinary Medicine headed up care for 9-year-old Sophie, the beloved pet of Karen Cortellino, pictured here with her son Alex Peña. Not long after the ablation procedure, Cortellino says the boxer was “back to her perky self.” (Image: John Donges/Penn Vet)
Cardiology resident Alexandra Crooks and cardiologist Anna Gelzer of the School of Veterinary Medicine headed up care for 9-year-old Sophie, the beloved pet of Karen Cortellino, pictured here with her son Alex Peña. Not long after the ablation procedure, Cortellino says the boxer was “back to her perky self.” (Image: John Donges/Penn Vet)For Karen Cortellino, her 9-year-old dog Sophie is more than just a companion.
“There’s this bumper sticker that says, ‘Rescue dogs: Who rescued who?’” says Cortellino, a physician from New Jersey. “That’s exactly how I feel.” Eight years ago, she adopted Sophie, a boxer, two weeks after the death of the family’s first boxer, and “she’s been Mommy’s baby ever since.”
A few months ago, however, Sophie’s star rose even higher: She became the first dog with a particular type of heart disease—arrhythmogenic right ventricle cardiomyopathy (ARVC)—to be treated with cardiac ablation.
Anna Gelzer, a cardiologist in Penn’s School of Veterinary Medicine, led Sophie through the procedure, together with cardiology resident Alexandra Crooks. But the equipment and expertise to perform an ablation, in which a high energy catheter tip burns tiny portions of damaged heart tissue to restore normal rhythms, wouldn’t have been possible without collaborators from just down the street. At the Perelman School of Medicine’s Translational Cardiac Electrophysiology Laboratory, Director Cory Tschabrunn and members of his team worked hand-in-hand with their veterinary colleagues to plan out and provide Sophie a procedure that mirrors the best that human medicine has to offer.
“This collaboration and this close distance between our hospitals allows us to be able to utilize the tremendous access to all this knowledge,” says Gelzer. “And from our experience with Sophie and other dogs to come, we may able to glean information that will be valuable to human medicine. It’s the best of both worlds.”
For Gelzer and Crooks, Sophie is a pilot case for a study now backed by two grants that will support cardiac mapping and ablation procedures for six additional dogs. Currently, cardiac ablation is only available for pet dogs in two other sites in the world, one in Italy and one in Ohio. Sophie’s case puts Penn Vet on the map. While the equipment necessary to perform ablations is costly, access to Penn Medicine’s translational electrophysiology lab has opened the possibility that Penn Vet may one day be able to provide committed dog owners a more durable alternative to medication for treating their pets’ arrhythmias.
A scary spell
ARVC is not an uncommon diagnosis in boxers. Some studies estimate as much as a quarter of the breed may have the inherited disease, which is also prevalent in American bulldogs. But Sophie’s heart was not top of mind in early July, when she had surgery to repair a torn ligament in her left knee. Two weeks later, Cortellino took her for a follow-up visit at their local veterinary hospital to have her stitches removed.
 Sophie’s diagnosis of ARVC meant she could suffer a life-threatening arrhythmia, despite starting medications to reduce that risk. (Image: John Donges/Penn Vet)
Sophie’s diagnosis of ARVC meant she could suffer a life-threatening arrhythmia, despite starting medications to reduce that risk. (Image: John Donges/Penn Vet)“Everything was great and literally we were just about walking out the door when Sophie collapsed,” Cortellino says.
Sophie received emergency care, was transferred to another veterinary facility with a cardiac department, and was soon diagnosed with ARVC. A strikingly similar condition affects roughly 1 in 1,000 humans. In both dogs and humans, the disease, which doesn’t manifest until adulthood, causes a deterioration of the tissues in the heart muscle, leading to occasional episodes when the heart beats very fast.
The condition increases the risk of sudden death. While drugs like beta blockers and sodium channel blockers can mitigate this risk, arrhythmias can sometimes break through these medications.
RELATED
Cutting-edge science moves to the clinic to help ‘our furry friends’ fight cancer
Going out of the box to learn to treat exotic creatures
Meaningful science, with students at the helm
Where ethics, welfare, and sustainability meet swine
Celebrating five years of working dogs at Penn
“It was kind of a somber picture when she was diagnosed,” Cortellino says. “She could have a fatal arrhythmia at any time: today, next month, next year, three years from now.”
Cortellino, capitalizing on her medical training, began researching alternative treatment options. In humans with a diagnosis similar to Sophie’s, the treatment of choice is an implantable cardiac defibrillator (ICD). But, as Gelzer explained to Cortellino when she reached out about this possibility, that option is not yet tenable for dogs.
“ICDs are designed to recognize human arrhythmias,” Gelzer says. “But they’re not able to distinguish the normal variation in heart rate that a dog is capable of from a life-threatening arrhythmia.”
An affectionate dog awaiting its owners’ return home for work, for example, might get so excited upon hearing a key turn in the door that its heart rate could jump from 40 to 200 beats per minute within the space of a few heartbeats. If that dog was outfitted with an ICD, the device might interpret the rate change as an arrhythmia and misfire, triggering a painful and possibly traumatic shock.
But Gelzer did have an alternative proposal for Cortellino, one that could address the underlying cause of Sophie’s heart condition. The only catch was that it had never been done in a dog with ARVC before.
Ideal expertise
When Cortellino emailed Gelzer, the timing was good. Gelzer had been thinking about options for curing cases like Sophie’s for many years. And she had the right kind of expertise to be considering that possibility.
In 2000, when Gelzer was a junior faculty member at Penn Vet, she worked with David Callans, an expert on cardiac electrophysiology at the School of Medicine. At the time, they collaborated on basic cardiac research, using pig models. Gelzer remembers wishing they could apply the technique of ablations to dogs, which develop heart conditions similar to humans.
Fast forward 15 years, after time away from Penn Vet in positions at Cornell and University of Liverpool, Gelzer returned and reconnected with Callans. His old basic research lab was no longer operating, but Gelzer continued to reach out to him for consultations from time to time, or attended rounds for human patients in his group.
This collaboration and this close distance between our hospitals allows us to be able to utilize the tremendous access to all this knowledge.Veterinary cardiologist Anna Gelzer of the School of Veterinary Medicine
Roughly a year ago, when discussing one of Gelzer’s cases, Callans connected her with Tschabrunn, who had recently set up his lab in Penn Medicine’s Smilow Center for Translational Research as part of the recently established Electrophysiology Translational Center of Excellence (EP-TCE) initiative, led by Francis Marchlinski, director of cardiac electrophysiology for Penn Medicine. Marchlinski and the Penn EP team have been pioneers in the evaluation and treatment of patients with inherited arrhythmia disorders like ARVC.
Tschabrunn’s primary research interests focus on the development of clinically relevant translational research models to elucidate the underlying pathophysiology and mechanisms of complex arrhythmias and the creation of new diagnostic and therapeutic technologies for treating cardiovascular diseases. He also performs, in close collaboration with Marchlinski and with support from the Winkelman Family Fund in Cardiovascular Innovation, clinical research in human patients with ARVC.
As such, Tschabrunn responded with enthusiasm upon hearing of Gelzer’s interest in pursuing ablations in dogs, particularly those with ARVC. The two struck up a collaboration that brought together the latest in technique and technology in cardiac electrophysiology with deep knowledge in veterinary cardiology.
“This was an exciting opportunity not only in terms of a research collaboration,” says Tschabrunn, “but we also had the chance to help a patient by combining our expertise and resources that are really only available at just a few institutions in the world.”
Not a bandage
Ablations are “routine care” for many cases of arrhythmias in people. “You approach the heart through the blood vessel, get in the right spot, and—with all the expertise and knowledge of the practitioner—you can find the damaged area and burn it,” says Gelzer. “And then maybe the patient doesn’t need to be on medications that can have side effects and are in some cases not that effective.”
Gelzer saw Sophie, a healthy dog aside from her heart condition, as an excellent candidate for an ablation. Cortellino, while a bit nervous about putting her beloved dog into uncharted medical territory, was comforted by Gelzer’s and Crooks’s clear expertise, their warm manner with Sophie, and their openness and honesty about the procedure’s upsides—and possible risks.
“I was a little nervous—a lot nervous—but we thought to ourselves, really, what’s our alternative?” Cortellino says. “As my son said, ‘Look Mom, at the very least, Sophie is contributing to the possible welfare of other dogs.’ So there was a small element of altruism in putting Sophie through this, in addition to hoping for a more definitive treatment for her condition.”
Before the surgery, the veterinarians gathered data on the patterns of Sophie’s arrhythmias using a device called a Reveal LINQ, implanted just beneath her skin. The LINQ—which is also used in humans—records a continuous electrocardiogram (ECG) as a loop recorder, storing abnormal rhythm strips for up to three years, giving clinicians a more complete picture of abnormal heart activity than a quick office visit ECG. That information was used during the procedure to zero in on the correct area of the heart to target with the ablation.
 Performing the mapping of Sophie’s heart and the ablation procedure was a team effort, involving experts from both the School of Veterinary Medicine and the Perelman School of Medicine. “I think the openness and enthusiasm for this type of multidisciplinary collaboration is a major strength of this University,” says Cory Tschabrunn (to right of Sophie, with black glasses around neck), who directs the translational electrophysiology lab where it happened. (Image: Anna Gelzer)
Performing the mapping of Sophie’s heart and the ablation procedure was a team effort, involving experts from both the School of Veterinary Medicine and the Perelman School of Medicine. “I think the openness and enthusiasm for this type of multidisciplinary collaboration is a major strength of this University,” says Cory Tschabrunn (to right of Sophie, with black glasses around neck), who directs the translational electrophysiology lab where it happened. (Image: Anna Gelzer)The morning of the procedure, Gelzer used her own car to drive Sophie the short distance from Penn Vet’s Ryan Hospital to the Smilow Center. When she brought the dog up to the lab, a full complement of experts awaited her: not only Crooks, Tschabrunn and his team, and Giacomo Gianotti, head of anesthesia at Ryan, but also two anesthesia residents, Penn Vet’s two other cardiology faculty, Marc Kraus and Mark Oyama, two other cardiology residents, a cardiology research intern, experts on the machines that were used in the procedure, veterinary nurses, and interested observers.
“The number of people we had in one room for one patient, it blows my mind,” says Gianotti. “Everyone had a specific role, and it took a lot of training and cooperation to get there.”
The procedure was long and complex, taking place in different stages. First, to locate the areas of unhealthy heart tissue that had been indicated by the ECG, the clinicians used an advanced mapping system based on GPS technology called CARTO.
“You put a patch on the bottom and top of the dog,” Gelzer says. “You then use those as your points of orientation as you advance the catheter and create a map of the inside of the heart. It’s great because you don’t have to use fluoroscopy, so nobody is exposed to X-rays.”
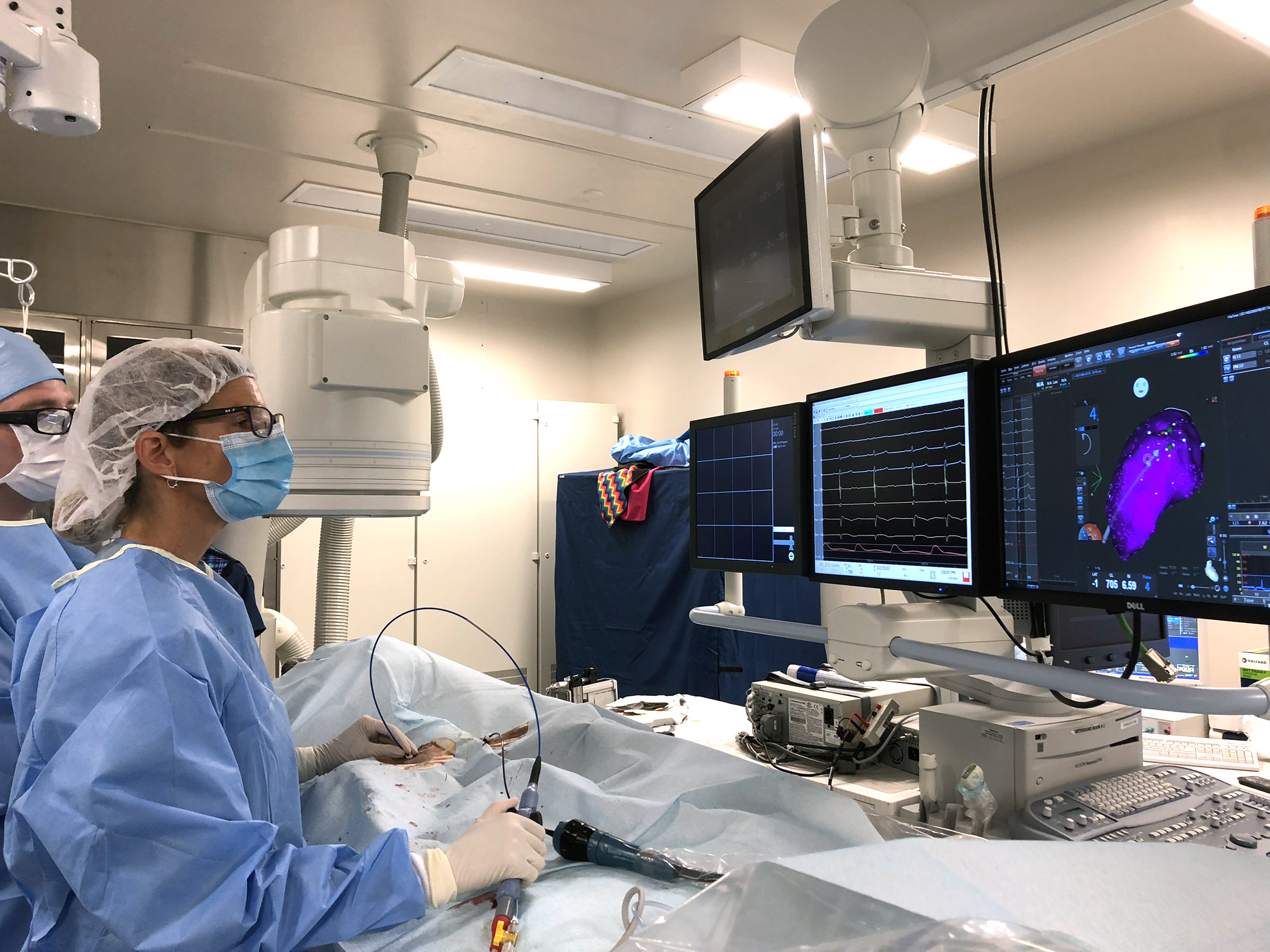 The technology Tschabrunn and Gelzer and colleagues used during the procedure mirrors that employed during a human intervention. At right, a map of Sophie’s heart guided the clinicians in making tiny burns to eliminate damaged heart tissue. (Image: Eva Larouche-Lebel)
The technology Tschabrunn and Gelzer and colleagues used during the procedure mirrors that employed during a human intervention. At right, a map of Sophie’s heart guided the clinicians in making tiny burns to eliminate damaged heart tissue. (Image: Eva Larouche-Lebel)The CARTO system maps the voltage of the heart tissue, a technique pioneered by Marchlinski and Callans nearly two decades ago and a continued area of Tschabrunn's research focus today in both the translational and clinical PE laboratories. Decreased voltage corresponds with diseased tissue. They confirmed these areas by artificially introducing extra heart beats into Sophie’s normal rhythm. But Sophie’s heart resisted these challenges, a sign that her disease was being kept in check by her medications.
The heart mapping and challenges did, however, allow the clinicians to reproduce the abnormal beats that they had seen on the ECG, giving them more evidence that they were targeting the right areas for ablation. Guided by that information, Tschabrunn used precisely directed radiofrequency to burn millimeter-sized portions of the tissue inside Sophie’s right ventricle, one of the lower chambers of the heart.
Throughout the several-hours-long procedure, Gelzer and Crooks sent texts with updates to Cortellino. “While it was nerve-wracking, I really felt that Sophie was in good hands,” she says.
And all went smoothly. “Sophie did amazing,” Gelzer says. “After we were done, we pulled the catheter out, she rested, and then went home the next day.”
Paving the way
Gelzer and Tschabrunn recently performed another ablation on a canine patient, and they are hopeful that the outcomes from the study will lay the groundwork for ablation to become a more routine option for dogs and their owners.
“My long-term hope for Penn Vet is that any arrhythmia that is potentially ablatable, we will be able to offer ablation therapy,” she says. “It’s not going to be the right option for every owner or dog, but with the right case, the right circumstance, it’s a very promising and rewarding treatment to be able to provide.”
Members of the team on both the veterinary and medical sides share enthusiasm about the information that canine patients will be able to lend to human medicine as well. “There is a lot we can learn about cardiac disease pathology from veterinary patients like Sophie,” says Tschabrunn. “It is extremely difficult or nearly impossible to model human-like inherited cardiac diseases and complex arrhythmias in the laboratory, but similar diseases can occur naturally in dogs. This provides us a unique opportunity to improve our understanding of these diseases and develop new treatments for human and veterinarian patients alike.”
 From Sophie’s case and others that follow, researchers hope to glean information that could benefit both human and veterinary patients in the future. (Image: Alexandra Crooks)
From Sophie’s case and others that follow, researchers hope to glean information that could benefit both human and veterinary patients in the future. (Image: Alexandra Crooks)This type of mutually beneficial exchange highlights the value of a One Health approach to medicine, one that takes advantage of the remarkable similarities between humans and our companion animals, says Oliver Garden, who heads the Department of Clinical Sciences and Advanced Medicine at Penn Vet.
“If ever there was a thrilling example of One Health in action, this is it,” says Garden. “Sophie’s case brings new heights to our department’s ethos of advanced medicine. And the work of such a transdisciplinary team, in this case involving members of our own esteemed faculty collaborating with experts at the Perelman School of Medicine, is nothing short of breathtaking.”
Tschabrunn concurs. “I think the openness and enthusiasm for this type of multi-disciplinary collaboration is a major strength of this University,” he says. “It is only possible in places like Penn, which brings together the expertise from faculty across so many diverse schools coupled with extraordinary facilities and resources all on a single campus. There’s always something incredible going on that you can be a part of.”
And Cortellino and her family are reaping the benefits: “Sophie is back to her perky self.”
Anna Gelzer is professor of cardiology at the University of Pennsylvania School of Veterinary Medicine.
Alexandra Crooks is a resident in cardiology at the University of Pennsylvania School of Veterinary Medicine.
Giacomo Gianotti is associate professor of clinical anesthesiology at the University of Pennsylvania School of Veterinary Medicine.
Oliver Garden is the Henry and Corinne R. Bower Professor of Medicine and chair of the Department of Clinical Sciences and Advanced Medicine at the University of Pennsylvania School of Veterinary Medicine.
Cory Tschabrunn is an instructor of medicine and director of the Translational Cardiac Electrophysiology Laboratory at the University of Pennsylvania Perelman School of Medicine.
David Callans is professor of medicine at the University of Pennsylvania Perelman School of Medicine.
Francis Marchlinksi is the Richard T. and Angela Clark President’s Distinguished Professor in the Department of Medicine at the University of Pennsylvania Perelman School of Medicine.
Homepage photo: A grateful Sophie offers affection to cardiology resident Alexandra Crooks at a follow-up appointment after her ablation procedure. (Image: John Donges/Penn Vet)
No comments:
Post a Comment