Cryo-electron microscopy opens a door to fight Epstein-Barr
The Epstein-Barr virus is one of the most widespread human viruses. Part of the herpesvirus family, it causes glandular fever (infectious mononucleosis), cancer and autoimmune diseases. At present, there is no treatment for infections caused by this virus. In work recently published in Nature Communications, scientists from the Institute for Research in Biomedicine (IRB Barcelona) and the Molecular Biology Institute of Barcelona (IBMB-CSIC) in Spain used cryo-electron microscopy (cryo-EM) to reveal the structure of a key protein, known as a portal, in the Epstein-Barr virus. Similarities between herpesviruses and tailed bacteriophages (viruses that infect bacteria) suggest that these two types of organism may be related. In a second paper published in the same journal, the team solved the structure of the portal protein in bacteriophage T7, using a combination of cryo-EM and X-ray crystallography. These results allowed them to infer how the Epstein-Barr virus portal works and may help in the development of a treatment for this virus.
In 2018, we brought you the news that high-resolution cryo-EM at eBIC had uncovered new information about a critical feature of the Herpes Simplex Virus. Cryo-EM has now worked its magic on the related Epstein-Barr virus, paving the way towards ways to defeat this untreatable virus.
The herpesvirus family is enormous and includes eight human pathogens. The Epstein-Barr virus infects B-cells (a type of white blood cell) and also the epithelial cells that make up skin and also line the inside of the throat, blood vessels and organs. It causes glandular fever (infectious mononucleosis) and can cause several kinds of cancer and autoimmune diseases.
All herpesviruses infect in a similar way. Once the virus has entered a cell and reached the nucleus, it releases its DNA. This DNA can lie dormant for many years until specific conditions trigger replication. When the virus replicates, the DNA is introduced into a new viral shell (capsid), forming a new virus capable of attacking other cells. The virus uses a protein called a portal for packaging its DNA into the viral capsid and to release it to the host cell during infection. As the portal plays a critical role in replication and infection, it makes an attractive target for the development of new anti-viral drugs.
The portal: an open and shut case?
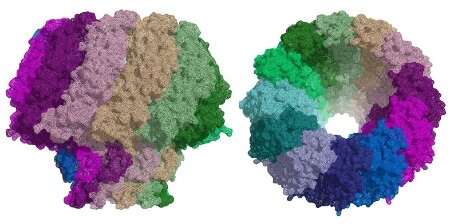
The similarities between the capsid structure and viral DNA packaging mechanism of herpesviruses and tailed bacteriophages suggest that they may be related. Although researchers have been able to determine the structure of portal proteins from bacteriophages successfully, the study of herpesvirus portals has been more challenging. Scientists from the Institute for Research in Biomedicine (IRB Barcelona) and the Molecular Biology Institute of Barcelona (IBMB-CSIC) have now used cryo-EM at eBIC to reveal the structure of the portal protein in the Epstein-Barr virus at a resolution of 3.5 Å.
In a second study, the same team used a combination of cryo-EM and X-ray crystallography to characterise the structure of the portal protein in bacteriophage T7. Their work illustrates the power of using these techniques in conjunction to solve challenging molecular structures.
The bacteriophage also uses its portal to package its DNA inside a pro-capsid. The tail components then assemble on the portal to make an infectious virus. The ejection conduit remains tightly sealed until infection, when the channel opens to deliver the DNA to the host cell. All of the portals analysed to date for the Caudovirales family of bacteriophages share common structural features.
In search of antivirals
Miquel Coll is head of the Structural Biology of Protein & Nucleic Acid Complexes and Molecular Machines Lab at IRB Barcelona and a professor at IBMB-CSIC. He says;
"Understanding the structure of the portal protein could aid the design of inhibitors for the treatment of herpesvirus infections such as Epstein-Barr. As this protein is only found in herpesviruses, these inhibitors would be virus-specific and may be less toxic for humans."
Cristina Machón and Montserrat Fàbrega, postdoctoral fellows at IRB Barcelona and IBMB-CSIC are first authors on both papers. They say that "solving the structure of the portal protein of bacteriophage T7 has allowed us to infer how the portal from Epstein-Barr virus works."
The drugs currently used to treat herpesvirus infections target the viral DNA polymerase. They are not very efficient, with serious side effects and the appearance of viral resistance after prolonged treatment. There is no specific treatment for the Epstein-Barr virus. Knowledge of the atomic structures of portal proteins will be extremely valuable, allowing the structure-driven design of compounds targeting their function—highly specific anti-virals that should cause fewer side effects.
No comments:
Post a Comment