by University of Kentucky
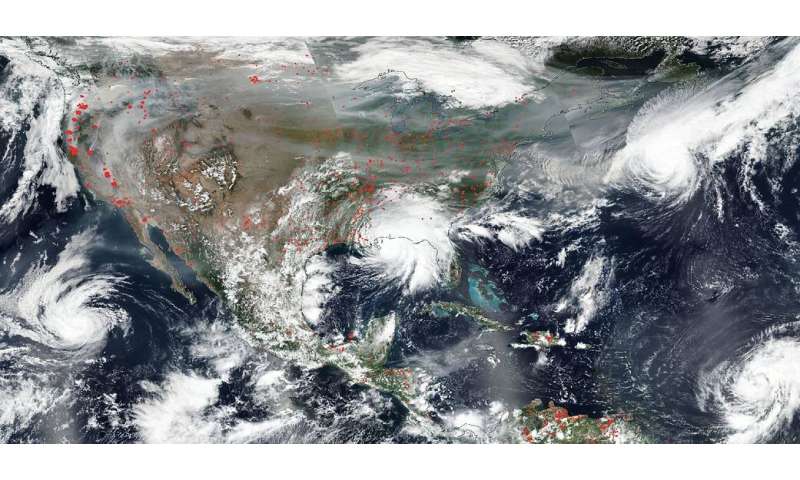
NOAA/NASA's Suomi NPP satellite captured this true-color image of the United States on Sep. 15, 2020 showing the fires in the West, the smoke from those fires drifting over the country, and several hurricanes converging with Sally making landfall. Credit: NOAA/NASA
Natural occurring wildfires create large smoke plumes that are transported several hundred miles away in the atmosphere exposing many people to pollutants that affect public health.
Every year, thousands of hectares of land are engulfed by forest fires across the globe. Just during the first three quarters of 2020, more than 2.6 million hectares in the Western United Sates have been consumed by fires. As the biomass in trees, bushes, grass, and peat are burned, large quantities of smoke, soot, and other pollutants are emitted to the atmosphere. The smoke can then rise several kilometers in altitude and spread across large continental regions, polluting the air of distant areas. For example, many residents in the states of California, Washington and Oregon have recently experienced the poor air quality of hazy smoke.
Chemistry professor Marcelo Guzman at the University of Kentucky leads a National Science Foundation research project, which is studying how emissions from biomass burning, including wildfires, change with time in the atmosphere to create new chemicals that impact the health of societies and the climate of Earth. Guzman, together with graduate student Sohel Rana, carefully studied in the laboratory the heterogeneous atmospheric chemistry of methoxyphenols, which are among the most abundant molecules emitted during biomass burning. The team highlighted that when methoxyphenols react at interfaces, i.e. such as on the surface of cloud and fog waters as well as aerosol particles from pollution, electron and proton transfer processes are favored to quickly convert aromatic molecules into highly water-soluble products.
'When you look at the mechanisms that these methoxyphenols undergo when exposed to background ozone gas and hydroxyl free radicals during atmospheric transport, you can start explaining the common observation of multifunctional carboxylic acids as abundant species in a lot of particles in the air we breathe. The report identifies unique reaction channels that can be used to distinguish the contribution of atmospheric processing of biomass burning emissions over other possible sources of multifunctional carboxylic acids,' said Prof. Guzman. 'The work is not only fundamentally interesting but identifies specific signatures for the daytime transformation of methoxyphenols emitted from forest fires as they age in the atmosphere.'
To do this, the researchers have used a special instrument in the laboratory that replicates the fast reaction between the methoxyphenols markers of biomass burning and ozone gas at the interface of air with micrometer size water droplets. They then vary the concentrations and acidity in the experiments to see how the interfacial chemistry changes for different conditions occurring in the environment.
'We are trying to understand the dominant transformations of the methoxyphenols from smoke in the atmosphere, determine their lifetime, and establish how they chemically evolve at interfaces,' said Prof. Guzman. 'We want to contribute new understanding of their impacts on human health and climate. Are the aged molecules more toxic? How do the structural changes of the molecules contribute to create particles that interact with sunlight affecting climate?'
A key finding of the work is that material released from forest fires can become more water soluble and likely toxic over the two weeks that smoke can be transported in the atmosphere. While in the air the methoxyphenols in smoke react with ozone and hydroxyl radicals to become oxidized and highly reactive. A person breathing in these reactive compounds can suffer oxidative damage of cells, especially in the respiratory track and lungs. In addition, these reactive compounds can make some people more prone to other health problems.
Prof. Guzman also states that characterizing the chemical processing of pollution from wildfires and domestic wood-burning can help to determine if the so-called brown carbon in soot emitted from fires contributes to absorb more heat from the sun or not. 'While the many small molecules in brown carbon can be quickly photobleached, the larger molecules are far more resistant, possibly contributing to warm up the atmosphere,' he said.
Explore further 'Four times more toxic': How wildfire smoke ages over time
More information: Find out more about this research: "Oxidation of Phenolic Aldehydes by Ozone and Hydroxyl Radicals at the Air–Water Interface" by Md. Sohel Rana and Marcelo I. Guzman, Journal of Physical Chemistry A, 2020, DOI: 10.1021/acs.jpca.0c05944.
Journal information: Journal of Physical Chemistry A
Provided by University of Kentucky
Natural occurring wildfires create large smoke plumes that are transported several hundred miles away in the atmosphere exposing many people to pollutants that affect public health.
Every year, thousands of hectares of land are engulfed by forest fires across the globe. Just during the first three quarters of 2020, more than 2.6 million hectares in the Western United Sates have been consumed by fires. As the biomass in trees, bushes, grass, and peat are burned, large quantities of smoke, soot, and other pollutants are emitted to the atmosphere. The smoke can then rise several kilometers in altitude and spread across large continental regions, polluting the air of distant areas. For example, many residents in the states of California, Washington and Oregon have recently experienced the poor air quality of hazy smoke.
Chemistry professor Marcelo Guzman at the University of Kentucky leads a National Science Foundation research project, which is studying how emissions from biomass burning, including wildfires, change with time in the atmosphere to create new chemicals that impact the health of societies and the climate of Earth. Guzman, together with graduate student Sohel Rana, carefully studied in the laboratory the heterogeneous atmospheric chemistry of methoxyphenols, which are among the most abundant molecules emitted during biomass burning. The team highlighted that when methoxyphenols react at interfaces, i.e. such as on the surface of cloud and fog waters as well as aerosol particles from pollution, electron and proton transfer processes are favored to quickly convert aromatic molecules into highly water-soluble products.
'When you look at the mechanisms that these methoxyphenols undergo when exposed to background ozone gas and hydroxyl free radicals during atmospheric transport, you can start explaining the common observation of multifunctional carboxylic acids as abundant species in a lot of particles in the air we breathe. The report identifies unique reaction channels that can be used to distinguish the contribution of atmospheric processing of biomass burning emissions over other possible sources of multifunctional carboxylic acids,' said Prof. Guzman. 'The work is not only fundamentally interesting but identifies specific signatures for the daytime transformation of methoxyphenols emitted from forest fires as they age in the atmosphere.'
To do this, the researchers have used a special instrument in the laboratory that replicates the fast reaction between the methoxyphenols markers of biomass burning and ozone gas at the interface of air with micrometer size water droplets. They then vary the concentrations and acidity in the experiments to see how the interfacial chemistry changes for different conditions occurring in the environment.
'We are trying to understand the dominant transformations of the methoxyphenols from smoke in the atmosphere, determine their lifetime, and establish how they chemically evolve at interfaces,' said Prof. Guzman. 'We want to contribute new understanding of their impacts on human health and climate. Are the aged molecules more toxic? How do the structural changes of the molecules contribute to create particles that interact with sunlight affecting climate?'
A key finding of the work is that material released from forest fires can become more water soluble and likely toxic over the two weeks that smoke can be transported in the atmosphere. While in the air the methoxyphenols in smoke react with ozone and hydroxyl radicals to become oxidized and highly reactive. A person breathing in these reactive compounds can suffer oxidative damage of cells, especially in the respiratory track and lungs. In addition, these reactive compounds can make some people more prone to other health problems.
Prof. Guzman also states that characterizing the chemical processing of pollution from wildfires and domestic wood-burning can help to determine if the so-called brown carbon in soot emitted from fires contributes to absorb more heat from the sun or not. 'While the many small molecules in brown carbon can be quickly photobleached, the larger molecules are far more resistant, possibly contributing to warm up the atmosphere,' he said.
Explore further 'Four times more toxic': How wildfire smoke ages over time
More information: Find out more about this research: "Oxidation of Phenolic Aldehydes by Ozone and Hydroxyl Radicals at the Air–Water Interface" by Md. Sohel Rana and Marcelo I. Guzman, Journal of Physical Chemistry A, 2020, DOI: 10.1021/acs.jpca.0c05944.
Journal information: Journal of Physical Chemistry A
Provided by University of Kentucky
No comments:
Post a Comment