Investigation Into the Origin of Elements in the Universe Yields New Insights
A key reaction in the slow neutron-capture process that forms elements occurs less frequently than previously thought.
The Science
The slow neutron-capture process (the s-process) is one of the nucleosynthesis processes that occurs in stars. It results in about half of the elements heavier than iron in the universe. Two important reactions involved in the s-process are Neon-22 (alpha, gamma) and Neon-22 (alpha, neutron). In these reactions, neutron-rich Neon-22 captures alpha-particles. The capture produces Magnesium-26 in an excited state, meaning it has received extra energy. It then releases energy by emitting either a gamma ray, leading to Magnesium-26 in a normal state, or a neutron, leading to Magnesium-25. The rates of both the Neon-22 (alpha, gamma) and Neon-22 (alpha, neutron) reactions have significant effects on the s-process. This affects the abundances of elements such as Selenium, Krypton, Rubidium, Strontium, and Zirconium.
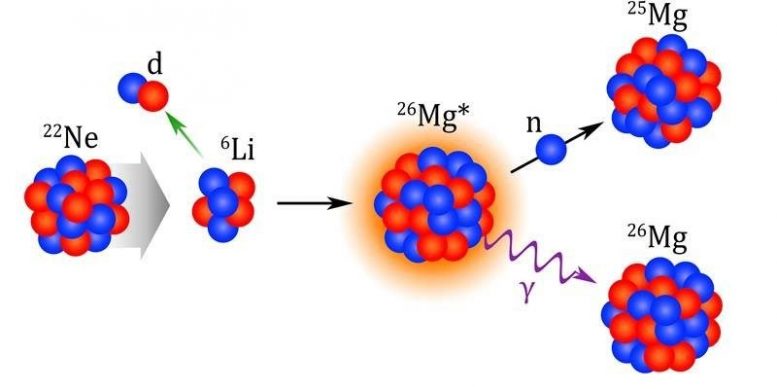
An isotope of Neon (22Ne) captures an alpha-particle (α) to create Magnesium-26 (26Mg) in an excited state. The excited Magnesium-26 then releases energy by emitting a gamma ray (γ), leading to Magnesium-26 or a neutron, leading to Magnesium-25. Credit: Image courtesy of Dustin Scriven, Texas A&M University
The Impact
Scientists are trying to answer the question, what is the origin of elements in the universe? The answer is extremely complex. It requires a collaborative effort by researchers in many fields and an enormous amount of experimental data. One part of answering this question is understanding the specific processes that create elements heavier than iron. Some of these elements form through particular nuclear reactions inside stars that involve neutron captures (the s-process). Neutrons are unstable and need to be produced continuously to fuel this process. Determining the intensities of neutron source reactions is important to understanding this nucleosynthesis scenario.
Summary
Two reactions have a strong influence on the neutron flux during the s-process, 22Ne(α, γ)26Mg and 22Ne(α, n)25Mg. The probabilities of these reactions occurring are difficult to measure directly because these probabilities (called reaction cross sections) are extremely low at the energies relevant for stellar nucleosynthesis. A team of nuclear physicists used two indirect methods to determine the probabilities for both reactions. Both methods used a 22-Neon beam produced at the Texas A&M University Cyclotron Institute. In one study, the team measured the likelihood for the most relevant excited states in 26-Magnesium to decay by alpha-particles. The other experiment involved direct measurements of neutron/gamma branching ratios for the same excited states. Combining these studies led researchers to a consistent conclusion: that the actual probability of the 22Ne(α, n)25Mg reaction occurring is lower than the widely accepted probability by a factor of three. This finding significantly changes the final s-process abundances of some elements, such as Selenium, Krypton, Rubidium, Strontium, and Zirconium.
References
Jayatissa, H., et al. Constraining the 22Ne(α,γ)26Mg and 22Ne(α,n)25Mg reaction rates using sub-Coulomb α-transfer reactions. Physics Letters B 802, 135267 (2020). [DOI: 10.1016/j.physletb.2020.135267]
Ota, S. et al. Decay properties of 22Ne + α resonances and their impact on s-process nucleosynthesis. Physics Letters B 802, 135256 (2020). [DOI: 10.1016/j.physletb.2020.135256]
Funding
This research was supported by the Department of Energy (DOE) Office of Science, Office of Nuclear Physics; by the National Nuclear Security Administration through the Center for Excellence in Nuclear Training and University Based Research (CENTAUR); and the Nuclear Solutions Institute at Texas A&M University. Two of the authors were also supported by The Welch Foundation. Three of the authors were also supported by the U.K. Science and Technology Facilities Council.
No comments:
Post a Comment