How ant teeth cut like a scalpel
Atomic-scale imaging reveals tiny animals use zinc to sharpen their miniature tools
IMAGE: ARUN DEVARAJ AT WORK IN THE LAB. view more
CREDIT: PACIFIC NORTHWEST NATIONAL LABORATORY
Ever wonder how tiny creatures can so easily slice, puncture, or sting? New research reveals that ants, worms, spiders, and other tiny creatures have a built-in set of tools that would be the envy of any carpenter or surgeon.
A recent study, published in the Nature journal Scientific Reports, shows for the first time how individual atoms of zinc are arranged to maximize cutting efficiency and maintain the sharpness of these exquisitely constructed tiny animal tools. A collaboration between a research team at the University of Oregon and the U.S. Department of Energy’s (DOE’s) Pacific Northwest National Laboratory (PNNL) revealed nature’s solution to enable tiny creatures to cut and puncture with relative ease.
When the ant bites
Consider the ant tooth. Yes, ants have teeth, as anyone who has ever stepped on an ant mound can attest. These specialized structures, technically called “mandibular teeth” because they are attached outside of their mouths, are made of a network of material that tightly binds individual atoms of zinc. The total effect is a mandible that packs more than 8 percent of the tooth weight with zinc.
These kinds of specialized critter tools have been a decades-long fascination for University of Oregon associate professor Robert Schofield, who led this study. His team of biophysicists has developed techniques to measure the hardness, elasticity, energy of fracture, abrasion resistance, and impact resistance on a miniature scale.
But they couldn’t actually see the structure of the materials that make up ant teeth and other microscopic animal tools, especially at the atomic scale. That’s where PNNL materials scientist Arun Devaraj and doctoral intern Xiaoyue Wang entered the picture. Devaraj is an expert in the use of a specialized microscope technique called atom probe tomography. He used a focused ion beam microscope to take a tiny needle sample from the tip of an ant tooth and then imaged that needle sample using atom probe tomography, allowing the team to identify how individual atoms are arranged near the tip of an ant tooth.
Using this technique, Devaraj and Wang recorded for the first time the nanoscale distribution of zinc atoms in the ant tooth.
“We could see that the zinc is uniformly distributed in the tooth, which was a surprise,” said Devaraj. “We were expecting the zinc to be clustered in nano-nodules.”
The research team estimated that, because these biomaterials can be sharper, they make it possible for the animals to use 60 percent or even less of the force that they would have to use if their tools were made of materials similar to that found in human teeth. Because less force is required, their smaller muscles spend less energy. These advantages may explain why every spider, ant, other insects, worms, crustaceans, and many other groups of organisms have these specialized tools.
CAPTION
Ant mandibles pack a powerful bite, thanks to embedded atoms of zinc.
CREDIT
Robert Schofield | University of Oregon
Ouch! Ant teeth at work
“Human engineers might also learn from this biological trick,” said Schofield. “The hardness of ant teeth, for example, increases from about the hardness of plastic to the hardness of aluminum when the zinc is added. While there are much harder engineering materials, they are often more brittle.”
Learning from nature is one way of understanding what makes materials stronger and more damage-resistant, added Devaraj. He is currently using a DOE Early Career Award to study, at the atomic scale, principles that make some materials strong and damage resistant. “By studying steel microstructure also at the atomic scale, we can better understand how altering the composition of materials changes its damage resistance, specifically stress corrosion resistance and behavior over time,” he said. “This is especially important for designing structures like nuclear power plants that need to withstand aging for many decades.”
The research study was supported by the National Science Foundation-supported research Center for Advanced Materials Characterization, a University of Oregon-based facility. A portion of the work was conducted at the Environmental Molecular Sciences Laboratory (EMSL), a DOE Office of Science user facility at PNNL in Richland, Washington.
JOURNAL
Scientific Reports
METHOD OF RESEARCH
Experimental study
ARTICLE TITLE
The homogenous alternative to biomineralization: Znand Mn‑rich materials enable sharp organismal “tools” that reduce force requirements
ARTICLE PUBLICATION DATE
1-Sep-2021
Zinc-infused proteins are the secret that allows scorpions, spiders and ants to puncture tough skin

Many small animals grow their teeth, claws and other "tools" out of materials that are filled with zinc, bromine and manganese, reaching up to 20% of the material's weight. My colleagues and I call these "heavy element biomaterials," and in a new paper, we suggest that these materials make it possible for animals to grow scalpel-sharp and precisely shaped tools that are resistant to breaking, deformation and wear.
Because of the small size of things like ant teeth, it has been hard for biologists to test how well the materials they are made of resist fractures, impacts and abrasions. My research group developed machines and methods to test these and other properties, and along with our collaborators, we studied their composition and molecular structure.
We examined ant mandible teeth and found that they are a smooth mix of proteins and zinc, with single zinc atoms attached to about a quarter of the amino acid units that make up the proteins forming the teeth. In contrast, calcified tools—like human teeth—are made of relatively large chunks of calcium minerals. We think the lack of chunkiness in heavy element biomaterials makes them better than calcified materials at forming smooth, precisely shaped and extremely sharp tools.
To evaluate the advantages of heavy element biomaterials, we estimated the force, energy and muscle size required for cutting with tools made of different materials. Compared with other hard materials grown by these animals, the wear-resistant zinc material enables heavily used tools to puncture stiff substances using only one-fifth of the force. The estimated advantage is even greater relative to calcified materials that—since they can't be nearly as sharp as heavy element biomaterials—can require more than 100 times as much force.
It's not surprising that materials that could make sharp tools would evolve in small animals. A tick and a wolf both need to puncture the same elk skin, but the wolf has vastly stronger muscles. The tick can make up for its tiny muscles by using sharper tools that focus force onto smaller regions.
But, like a sharp pencil tip, sharper tool tips break more easily. The danger of fracture is made even worse by the tendency for small animals to extend their reach using long thin tools—like those pictured above. And a chipped claw or tooth may be fatal for a small animal that doesn't have the strength to cut with blunted tools.
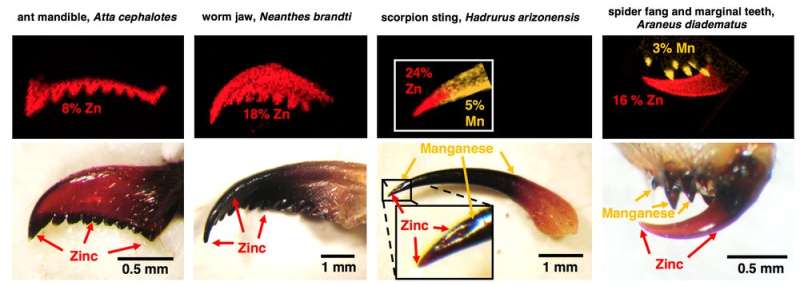
But we found that heavy element biomaterials are also particularly hard and damage-resistant.
From an evolutionary perspective, these materials allow smaller animals to consume tougher foods. And the energy saved by using less force during cutting can be important for any animal. These advantages may explain the widespread use of heavy element biomaterials in nature—most ants, many other insects, spiders and their relatives, marine worms, crustaceans and many other types of organisms use them.
While my team's research has clarified the advantages of heavy element biomaterials, we still don't know exactly how zinc and manganese harden and protect the tools.
One possibility is that a small fraction of the zinc, for example, forms bridges between proteins, and these cross-links stiffen the material—like crossbeams stiffen a building. We also think that when a fang bangs into something hard, these zinc cross-links may break first, absorbing energy to keep the fang itself from chipping.
We speculate that the abundance of extra zinc is a ready supply for healing the material by quickly reestablishing the broken zinc-histidine cross-links between proteins.
The potential that these materials are self-healing makes them even more interesting, and our team's next step is to test this hypothesis. Eventually we may find that self-healing or other features of heavy element biomaterials could lead to improved materials for things like small medical devices
Atomic-scale imaging reveals ants use zinc to sharpen their teeth
Provided by The Conversation
This article is republished from The Conversation under a Creative Commons license. Read the original article.![]()
No comments:
Post a Comment