A new particle accelerator aims to unlock secrets of bizarre atomic nuclei
The Facility for Rare Isotope Beams will help physicists learn how elements form in the distant environs of space
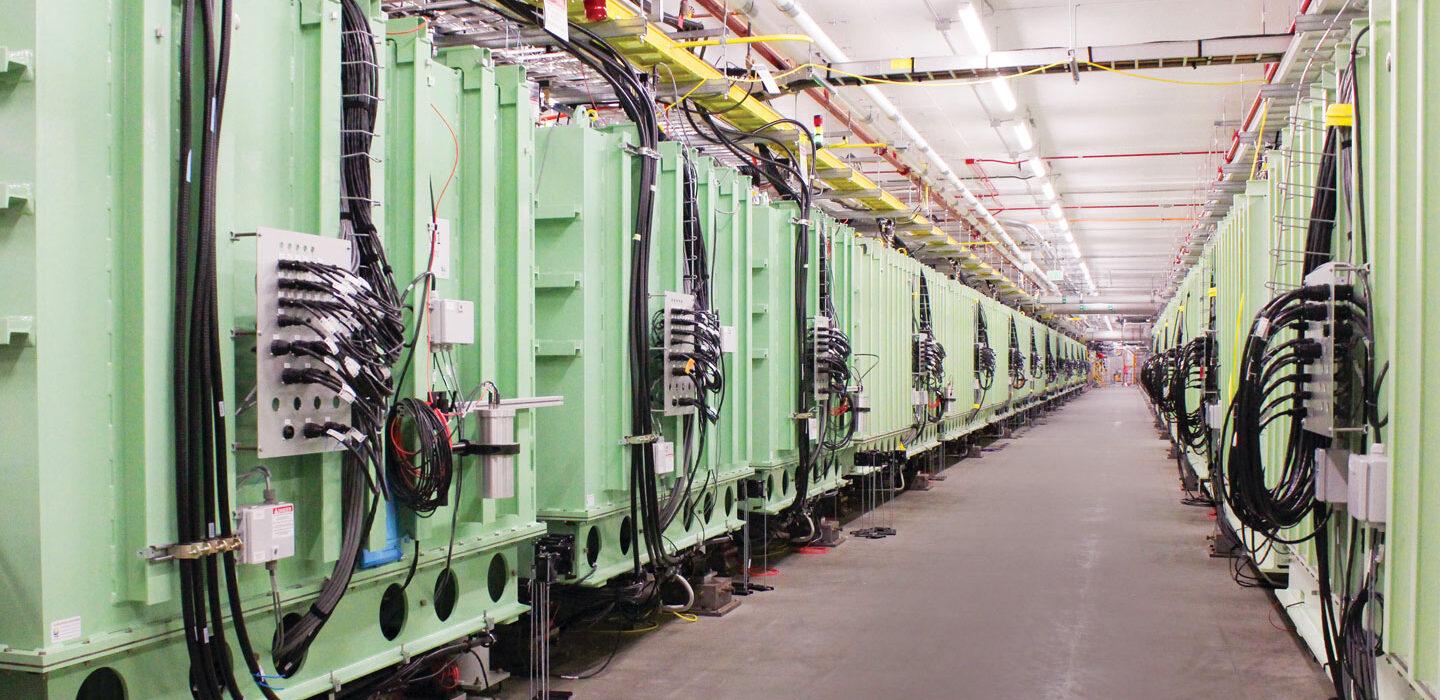
When it switches on in early 2022, the Facility for Rare Isotope Beams’ particle accelerator (shown) will accelerate beams of ions to about half the speed of light.
By Emily Conover
Inscribed on an Italian family’s 15th century coat of arms and decorating an ancient Japanese shrine, the Borromean rings are symbolically potent. Remove one ring from the trio of linked circles and the other two fall apart. It’s only when all three are entwined that the structure holds. The rings have represented the concepts of unity, the Christian Holy Trinity and even certain exotic atomic nuclei.
A rare variety, or isotope, of lithium has a nucleus that is made of three conjoined parts. Lithium-11’s nucleus is separated into a main cluster of protons and neutrons flanked by two neutrons, which form a halo around the core. Remove any one piece and the trio disbands, much like the Borromean rings.
Not only that, lithium-11’s nucleus is enormous. With its wide halo, it is the same size as a lead nucleus, despite having nearly 200 fewer protons and neutrons. The discovery of lithium-11’s expansive halo in the mid-1980s shocked scientists (SN: 8/20/88, p. 124), as did its Borromean nature. “There wasn’t a prediction of this,” says nuclear theorist Filomena Nunes of Michigan State University in East Lansing. “This was one of those discoveries that was like, ‘What? What’s going on?’ ”

Remove one of the three Borromean rings and the whole structure falls apart. Some atomic nuclei have the same property.
T. TIBBITTS
Lithium-11 is just one example of what happens when nuclei get weird. Such nuclei, Nunes says, “have properties that are mind-blowing.” They can become distorted into unusual shapes, such as a pear (SN: 6/15/13, p. 14). Or they can be sheathed in a skin of neutrons — like a peel on an inedible nuclear fruit (SN: 6/5/21, p. 5).
A new tool will soon help scientists pluck these peculiar fruits from the atomic vine. Researchers are queuing up to use a particle accelerator at Michigan State to study some of the rarest atomic nuclei. When it opens in early 2022, the Facility for Rare Isotope Beams, or FRIB (pronounced “eff-rib”), will strip electrons off of atoms to make ions, rev them up to high speeds and then send them crashing into a target to make the special nuclei that scientists want to study.
Experiments at FRIB will probe the limits of nuclei, examining how many neutrons can be crammed into a given nucleus, and studying what happens when nuclei stray far from the stable configurations found in everyday matter. With FRIB data, scientists aim to piece together a theory that explains the properties of all nuclei, even the oddballs. Another central target: pinning down the origin story for chemical elements birthed in the extreme environments of space.
And if scientists are lucky, new mind-blowing nuclear enigmas, perhaps even weirder than lithium-11, will emerge. “We’re going to have a new look into an unexplored territory,” says nuclear physicist Brad Sherrill, scientific director of FRIB. “We think we know what we’ll find, but it’s unlikely that things are going to be as we expect.”
Curious halo
Lithium-11’s nucleus has a center packed with protons and neutrons, surrounded by two neutrons in a broad halo. If one of those three components is removed, the nucleus can’t stay bound, what’s known as a Borromean nucleus.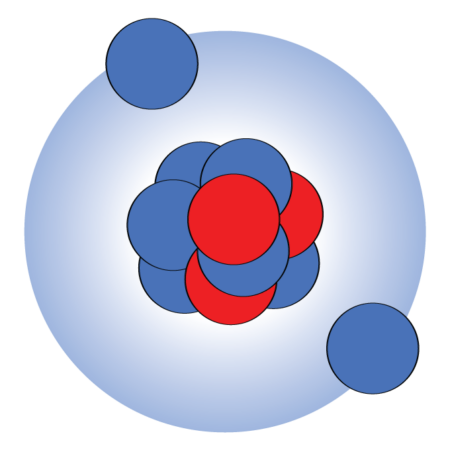
A new tool will soon help scientists pluck these peculiar fruits from the atomic vine. Researchers are queuing up to use a particle accelerator at Michigan State to study some of the rarest atomic nuclei. When it opens in early 2022, the Facility for Rare Isotope Beams, or FRIB (pronounced “eff-rib”), will strip electrons off of atoms to make ions, rev them up to high speeds and then send them crashing into a target to make the special nuclei that scientists want to study.
Experiments at FRIB will probe the limits of nuclei, examining how many neutrons can be crammed into a given nucleus, and studying what happens when nuclei stray far from the stable configurations found in everyday matter. With FRIB data, scientists aim to piece together a theory that explains the properties of all nuclei, even the oddballs. Another central target: pinning down the origin story for chemical elements birthed in the extreme environments of space.
And if scientists are lucky, new mind-blowing nuclear enigmas, perhaps even weirder than lithium-11, will emerge. “We’re going to have a new look into an unexplored territory,” says nuclear physicist Brad Sherrill, scientific director of FRIB. “We think we know what we’ll find, but it’s unlikely that things are going to be as we expect.”
Curious halo
Lithium-11’s nucleus has a center packed with protons and neutrons, surrounded by two neutrons in a broad halo. If one of those three components is removed, the nucleus can’t stay bound, what’s known as a Borromean nucleus.

T. TIBBITTS
Exploring instability
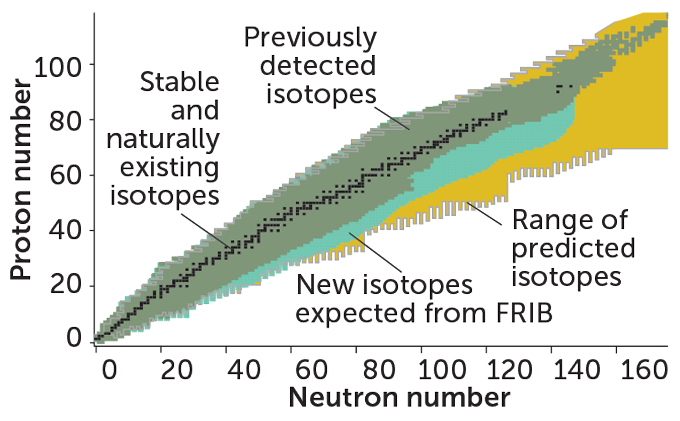
Atomic nuclei come in a dizzying number of varieties. Scientists have discovered 118 chemical elements, distinguished by the number of protons in their nuclei (SN: 1/19/19, p. 18). Each of those elements has a variety of isotopes, different versions of the element formed by switching up the number of neutrons inside the nucleus. Scientists have predicted the existence of about 8,000 isotopes of known elements, but only about 3,300 have made an appearance in detectors. Researchers expect FRIB will make a sizable dent in the missing isotopes. It may identify 80 percent of possible isotopes for all the elements up through uranium, including many never seen before.
The most familiar nuclei are those of the roughly 250 isotopes that are stable: They don’t decay to other types of atoms. The ranks of stable isotopes include the nitrogen-14 and oxygen-16 in the air we breathe and the carbon-12 found in all known living things. The number following the element’s name indicates the total number of protons and neutrons in the nucleus.
Stable nuclei have just the right combination of protons and neutrons. Too many or too few neutrons causes a nucleus to decay, sometimes slowly over billions of years, other times in mere fractions of a second (SN: 3/2/19, p. 32). To understand what goes on inside these unstable nuclei, scientists study them before they decay. In general, as the proton-neutron balance gets more and more off-kilter, a nucleus gets further from stability, and its properties tend to get stranger.
Such exotic specimens test the limits of scientists’ theories of the atomic nucleus. While a given theory might correctly explain nuclei that are near stability, it may fail for more unusual nuclei. But physicists want a theory that can explain the most unusual to the most banal.
“We would like to understand how the atomic nucleus is built, how it works,” says theoretical nuclear physicist Witold Nazarewicz, FRIB’s chief scientist.
Accelerating beams of ions in FRIB is like herding cats.
In the beginning, “it’s just a gaggle of cats,” says Thomas Glasmacher, FRIB’s laboratory director. The cats meander this way or that, but if you can nudge the unruly bunch in a particular direction — maybe you open a can of cat food — then the cats start moving together, despite their natural tendency to wander. “Pretty soon, it’s a stream of cats,” he says.
In FRIB’s case, the cats are ions — atoms with some or all of their electrons stripped off. And rather than cat food, electromagnetic forces get them moving en masse.
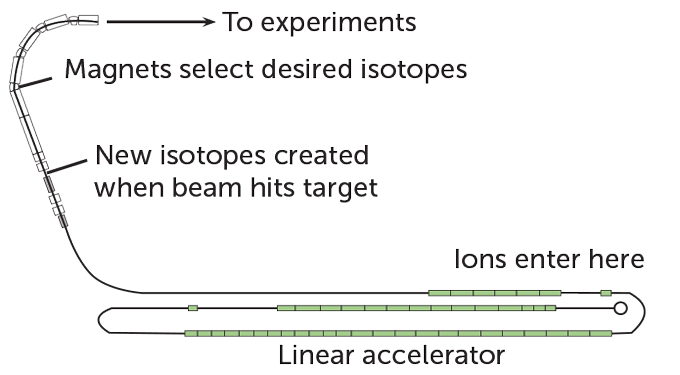
The journey starts in one of FRIB’s two ion sources, where elements are vaporized and ionized. After some initial acceleration to get the ions moving, the beam enters the linear accelerator, which is what sets the particles really cruising. The linear accelerator looks like a scaled-down freight train — a line of 46 boxes the color of pistachio ice cream, each about 2.5 meters tall, of varying lengths. But the accelerator sends the beam moving much faster than a cargo-filled train — up to about half the speed of light.
Within the green boxes, called cryomodules, superconducting cavities are cooled to just a few kelvins, a smidge above absolute zero. At those temperatures, the cavities can accelerate the ions using rapidly oscillating electromagnetic fields. The chain of pistachio modules wends around the facility in the shape of a paper clip, a contortion necessary so that the approximately 450-meter-long accelerator fits in the 150-meter-long tunnel that houses it.
When the beam is fully accelerated, it’s slammed into a graphite target. That hard hit knocks protons and neutrons off the nuclei of the incoming ions, forming new, rarer isotopes. Then, the specific one that a scientist wants to study is separated from the riffraff by magnets that redirect particles based on their mass and electric charge. The particles of interest are then sent to the experimental area, where scientists can use various detectors to study how the particles decay, measure their properties or determine what reactions they undergo.
Up to speed
FRIB’s accelerator is bent into a paper clip shape to fit the full 450-meter length of the apparatus in the tunnel where it is housed. Forty-six cryomodules (green boxes) contain superconducting cavities that accelerate particles. Once the ions are accelerated, they are slammed into a target to create new isotopes. Farther down the line, magnets separate out the specific isotopes that scientists want to study.
T. TIBBITTS
The energy of FRIB’s beam is carefully selected for producing rare isotopes. Too much energy would blow the nuclei apart when they collide with the target. So FRIB is designed to reach less than a hundredth the energy of the Large Hadron Collider at CERN near Geneva, the world’s most energetic accelerator.
Instead, the new accelerator’s potential rests on its juiced-up intensity: Essentially, it has lots and lots of particles in its beam. For example, FRIB will be able to slam 50 trillion uranium ions per second into its target. As a result, it will produce more intense streams of rare isotopes than its predecessors could.
For isotopes that are relatively easily produced, FRIB will churn out about a trillion per second; plenty to study. That opens prospects for scrutinizing isotopes that are more difficult to make. Those isotopes might pop up once a week in FRIB, but that’s still much more often than in a weaker beam. It’s like a case of low water pressure in the bathroom: “You can’t have a shower if it’s just trickling,” says Nunes, who is one of the leaders of a coalition of theoretical physicists supporting research at FRIB. Now, “FRIB is going to come in with a fire hose.”
The energy of FRIB’s beam is carefully selected for producing rare isotopes. Too much energy would blow the nuclei apart when they collide with the target. So FRIB is designed to reach less than a hundredth the energy of the Large Hadron Collider at CERN near Geneva, the world’s most energetic accelerator.
Instead, the new accelerator’s potential rests on its juiced-up intensity: Essentially, it has lots and lots of particles in its beam. For example, FRIB will be able to slam 50 trillion uranium ions per second into its target. As a result, it will produce more intense streams of rare isotopes than its predecessors could.
For isotopes that are relatively easily produced, FRIB will churn out about a trillion per second; plenty to study. That opens prospects for scrutinizing isotopes that are more difficult to make. Those isotopes might pop up once a week in FRIB, but that’s still much more often than in a weaker beam. It’s like a case of low water pressure in the bathroom: “You can’t have a shower if it’s just trickling,” says Nunes, who is one of the leaders of a coalition of theoretical physicists supporting research at FRIB. Now, “FRIB is going to come in with a fire hose.”

The FRIB cryogenic plant makes liquid helium to cool components of FRIB’s accelerator that rely on superconductors, which conduct electricity without resistance at temperatures just above absolute zero. MICHIGAN STATE UNIV.
Dripping with neutrons
That fire hose will also come in handy for pinpointing a crucial boundary known as the neutron drip line.
Try to stuff too many neutrons in a nucleus, and it will decay almost immediately by spitting out a neutron. Imagine a greedy chipmunk with its cheeks so full of nuts that when it tries to shove in one more, another nut pops right back out. The threshold at which nuclei decay in this way marks the ultimate limits for bound nuclei. On a chart of the known elements and their isotopes, this boundary traces out a line, the neutron drip line. So far, scientists know the location of this crucial demarcation up through, at most, the 10th element on the periodic table, neon.
“FRIB is going to be the only way to go heavier and far enough out to define that drip line,” says nuclear physicist Heather Crawford of Lawrence Berkeley National Laboratory in California. FRIB is expected to determine the neutron drip line up to the 30th element, zinc, and maybe even farther.
Nuclear limits
Scientists have discovered a slew of isotopes of chemical elements (green). FRIB is expected to find new ones (turquoise) within the full range of predicted isotopes (gold). The neutron drip line, the bottom edge of the colored region, marks the limits of nuclei, but scientists don’t know exactly where it lies.
Landscapes of isotopes
FRIB
Near that drip line, where neutrons greatly outnumber protons, is where nuclei get especially strange. Lithium-11, with its capacious halo, sits right next to the drip line. Crawford focuses on magnesium isotopes that are close to the drip line. The most common stable magnesium isotope has 12 protons and 12 neutrons. Crawford’s main target, magnesium-40, has 12 protons and more than double that number of neutrons — 28 — in its nucleus.
“That’s right out at the limits of existence,” Crawford says. Out there, theories that predict the properties of nuclei are no longer reliable. Theoretical physicists can’t always be sure what size and shape a given nucleus in this realm might be, or even whether it qualifies as a bound nucleus. A given theory might also fall short when predicting how much energy is needed to bump the nucleus into its various energized states. The spacing of these energy levels acts as a kind of fingerprint of an atomic nucleus, one that’s highly sensitive to the details of the nucleus’ shape and other properties.
Sure enough, magnesium-40 behaves unexpectedly, Crawford and colleagues reported in 2019 in Physical Review Letters. While theories predicted its energy levels would match those of magnesium isotopes with slightly fewer neutrons, magnesium-40’s energy levels were significantly lower than its neighbors’.
In August, Crawford learned that she will be one of the first scientists to use FRIB. Two experiments she and colleagues proposed were selected for the first round of about 30 experiments to take place over FRIB’s first two years. She’ll take a closer look at magnesium-40, which, like lithium-11, has a Borromean nucleus. Crawford now aims to determine if her chosen isotope also has a haloed nucleus. That’s one possible explanation for magnesium-40’s oddness. Despite the fact that nuclei with halos have been known for decades, theories still can’t reliably predict which nuclei will be festooned with them. Understanding magnesium-40 could help scientists firm up their accounting of nuclei’s neutron adornments.
Nucleus possibilities
Unstable magnesium-40 has a nucleus packed with many more neutrons (blue) than the more common, stable magnesium-24, although both have the same number of protons (red). Scientists want to know if magnesium-40 has a typical nucleus or one with a large neutron halo.
Magnesium-24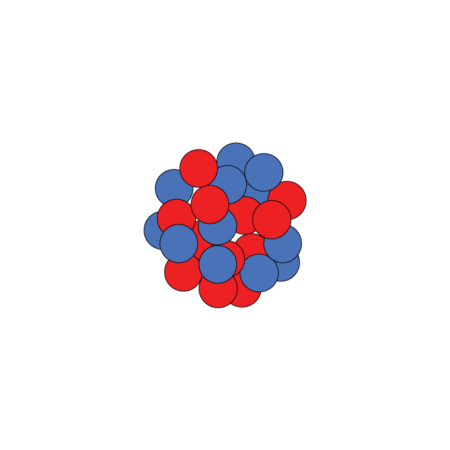
Standard magnesium-40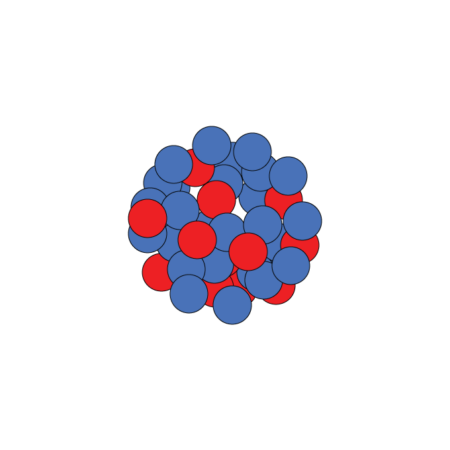
Magnesium-40 with halo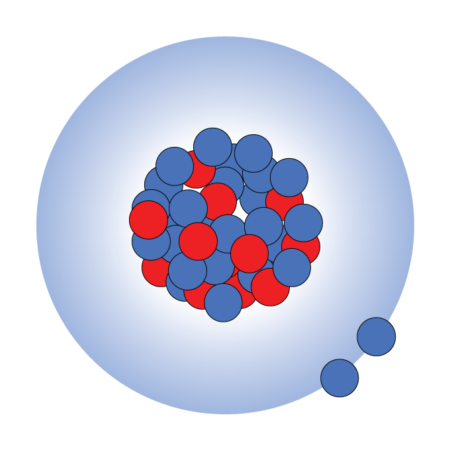
ALL IMAGES: T. TIBBITTS
Near that drip line, where neutrons greatly outnumber protons, is where nuclei get especially strange. Lithium-11, with its capacious halo, sits right next to the drip line. Crawford focuses on magnesium isotopes that are close to the drip line. The most common stable magnesium isotope has 12 protons and 12 neutrons. Crawford’s main target, magnesium-40, has 12 protons and more than double that number of neutrons — 28 — in its nucleus.
“That’s right out at the limits of existence,” Crawford says. Out there, theories that predict the properties of nuclei are no longer reliable. Theoretical physicists can’t always be sure what size and shape a given nucleus in this realm might be, or even whether it qualifies as a bound nucleus. A given theory might also fall short when predicting how much energy is needed to bump the nucleus into its various energized states. The spacing of these energy levels acts as a kind of fingerprint of an atomic nucleus, one that’s highly sensitive to the details of the nucleus’ shape and other properties.
Sure enough, magnesium-40 behaves unexpectedly, Crawford and colleagues reported in 2019 in Physical Review Letters. While theories predicted its energy levels would match those of magnesium isotopes with slightly fewer neutrons, magnesium-40’s energy levels were significantly lower than its neighbors’.
In August, Crawford learned that she will be one of the first scientists to use FRIB. Two experiments she and colleagues proposed were selected for the first round of about 30 experiments to take place over FRIB’s first two years. She’ll take a closer look at magnesium-40, which, like lithium-11, has a Borromean nucleus. Crawford now aims to determine if her chosen isotope also has a haloed nucleus. That’s one possible explanation for magnesium-40’s oddness. Despite the fact that nuclei with halos have been known for decades, theories still can’t reliably predict which nuclei will be festooned with them. Understanding magnesium-40 could help scientists firm up their accounting of nuclei’s neutron adornments.
Nucleus possibilities
Unstable magnesium-40 has a nucleus packed with many more neutrons (blue) than the more common, stable magnesium-24, although both have the same number of protons (red). Scientists want to know if magnesium-40 has a typical nucleus or one with a large neutron halo.
Magnesium-24

Standard magnesium-40

Magnesium-40 with halo

ALL IMAGES: T. TIBBITTS
Elemental origins
Physicists want to be able to poke around, like mechanics under the hood, to understand the cosmic nuclear reactions that make the universe go. “Nuclear physics is like the engine of a sports car. It’s what happens in the engine that determines how well the car performs,” says nuclear physicist Ani Aprahamian of the University of Notre Dame in Indiana.
The cosmos powered by that engine can be a violent place for nuclei, punctuated with dramatic stellar explosions and extreme conditions, including matter crammed into ultratight quarters by crushing gravity. These environments beget wonders of nuclear physics unlike those normally seen on Earth. FRIB will let scientists get a glimpse at some of those processes.
For example, physicists think that certain neutron-rich environments are the cauldron where many of the universe’s chemical elements are cooked. This cosmic connection allowed nuclear physicist Jolie Cizewski to make good on a childhood dream.
When Cizewski was a little girl, she caught the astronomy bug, she says. “I decided I was going to become an astronomer so I could go into space.” It might seem that she took a left turn from her childhood obsession. She never made it to orbit and she didn’t become an astronomer.
But echoes of that childhood dream now anchor her research. Instead of peering at the stars with a telescope, she’ll soon be using FRIB to reveal secrets of the cosmos.
Cizewski, of Rutgers University in New Brunswick, N.J., is working to unveil details of the cosmic nuclear reactions responsible for the nuclei that surround us. “I’m trying to understand how the elements, in particular those heavier than iron, have been synthesized,” she says.
Many of the elements around us — and in us — formed within stars. As large stars age, they fuse progressively larger atomic nuclei together in their cores, creating elements farther along the periodic table — oxygen, carbon, neon and others. But the process halts at iron. The rest of the elements must be born another way.
A process called the rapid neutron capture process, or r-process, is responsible for many of those other elements found in nature. In the r-process, atomic nuclei quickly soak up neutrons and bulk up to large masses. The neutronfest is interspersed with radioactive decays that form new elements. The sighting of two neutron stars merging in 2017 revealed that such collisions are one place where the r-process occurs (SN: 11/11/17, p. 6). But scientists suspect it might happen in other cosmic locales as well (SN: 6/8/19, p. 10).
Cizewski and colleagues are studying an abbreviated form of the r-process that might thrive in supernovas, which may not have enough oomph for the full r-process. The team has zeroed in on germanium-80, which plays a pivotal role in the weak r-process. Physicists want to know how likely this nucleus is to capture another neutron to become germanium-81. At FRIB, Cizewski will slam a beam of germanium-80 into deuterium, which has one proton and one neutron in its nucleus. Knowing how often germanium-80 captures the neutron will help scientists nail down the neutron-slurping chain of the weak r-process, wherever it might crop up.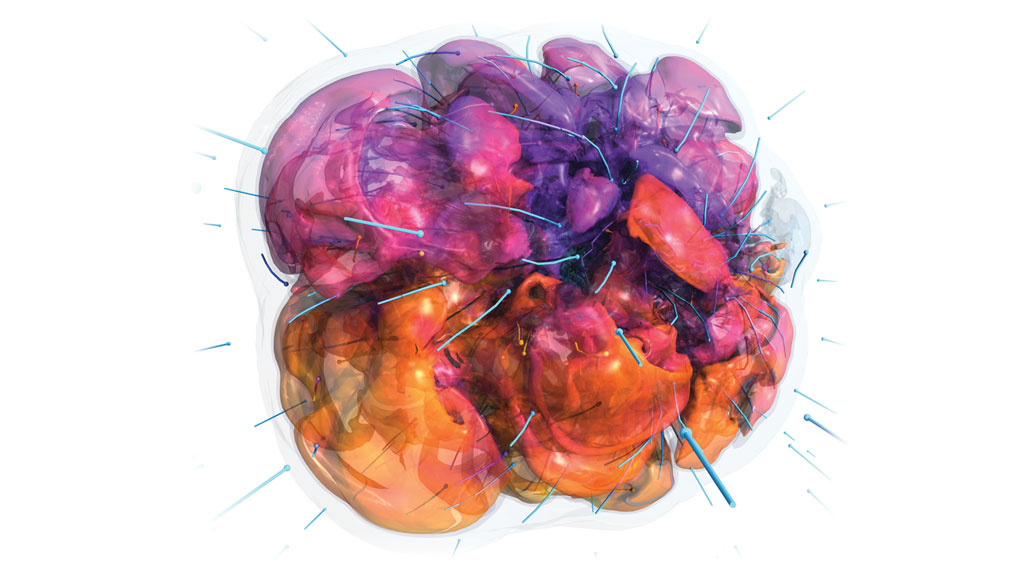
The cosmos powered by that engine can be a violent place for nuclei, punctuated with dramatic stellar explosions and extreme conditions, including matter crammed into ultratight quarters by crushing gravity. These environments beget wonders of nuclear physics unlike those normally seen on Earth. FRIB will let scientists get a glimpse at some of those processes.
For example, physicists think that certain neutron-rich environments are the cauldron where many of the universe’s chemical elements are cooked. This cosmic connection allowed nuclear physicist Jolie Cizewski to make good on a childhood dream.
When Cizewski was a little girl, she caught the astronomy bug, she says. “I decided I was going to become an astronomer so I could go into space.” It might seem that she took a left turn from her childhood obsession. She never made it to orbit and she didn’t become an astronomer.
But echoes of that childhood dream now anchor her research. Instead of peering at the stars with a telescope, she’ll soon be using FRIB to reveal secrets of the cosmos.
Cizewski, of Rutgers University in New Brunswick, N.J., is working to unveil details of the cosmic nuclear reactions responsible for the nuclei that surround us. “I’m trying to understand how the elements, in particular those heavier than iron, have been synthesized,” she says.
Many of the elements around us — and in us — formed within stars. As large stars age, they fuse progressively larger atomic nuclei together in their cores, creating elements farther along the periodic table — oxygen, carbon, neon and others. But the process halts at iron. The rest of the elements must be born another way.
A process called the rapid neutron capture process, or r-process, is responsible for many of those other elements found in nature. In the r-process, atomic nuclei quickly soak up neutrons and bulk up to large masses. The neutronfest is interspersed with radioactive decays that form new elements. The sighting of two neutron stars merging in 2017 revealed that such collisions are one place where the r-process occurs (SN: 11/11/17, p. 6). But scientists suspect it might happen in other cosmic locales as well (SN: 6/8/19, p. 10).
Cizewski and colleagues are studying an abbreviated form of the r-process that might thrive in supernovas, which may not have enough oomph for the full r-process. The team has zeroed in on germanium-80, which plays a pivotal role in the weak r-process. Physicists want to know how likely this nucleus is to capture another neutron to become germanium-81. At FRIB, Cizewski will slam a beam of germanium-80 into deuterium, which has one proton and one neutron in its nucleus. Knowing how often germanium-80 captures the neutron will help scientists nail down the neutron-slurping chain of the weak r-process, wherever it might crop up.

Nuclear physics goes extreme in supernovas (computer simulation shown below) and similar environments. New elements and exotic isotopes may be formed in the tumult.
ADAM BURROWS/PRINCETON UNIV., JOE INSLEY AND SILVIO RIZZI/ARGONNE NATIONAL LAB
A Borromean bent
Like the interlinked Borromean rings, different facets of nuclear physics are closely entwined, from mysteries of the cosmos to the inner workings of nuclei. The exotic nuclei that FRIB cooks up could also allow physicists to tap into the very bedrock of physics by testing certain fundamental laws of nature. And there’s a practical side to the facility as well. Scientists could collect some of the isotopes FRIB produces for use in medical procedures, for example.
Physicists are ready for surprises. “Every time we build such a facility, new discoveries come and breakthroughs in science come,” Nazarewicz says. Like the 1980s discovery of lithium-11’s Borromean nucleus, scientists may find something totally unexpected.
Icing on the cake
Along with studying atomic nuclei at extremes and exploring nuclear physics of the stars, scientists hope to use FRIB to make progress in two other key areas.

Harvesting useful nuclei
Scientists plan to collect isotopes produced in FRIB for societal applications. In medicine, for example, certain isotopes, such as terbium-149, can be used for radiation treatment or medical imaging.
When this isotope of the rare earth metal terbium decays, it can emit alpha particles (helium nuclei) that can kill cancer cells. Its half-life of 4.1 hours is in a sweet spot: fast enough to have an effect — it doesn’t take hundreds of years to decay — but not so fast that it’s gone within seconds, before it can do its work.

Testing laws of nature
Scientists plan to check certain physics rules, for example, the idea that matter and antimatter behave as mirror images. Certain hypothetical physics effects could cause particles to flout this rule, and that could help explain why there’s more matter than antimatter in the universe.
Effects that could make matter and antimatter behave differently might also cause electric charge in atoms to separate, with slightly more positive charge on one side of the atom and more negative on the other. In most atoms, this separation may be too tiny to measure. But in radium-225, which has a pear-shaped nucleus, the effect would be stronger, as the nucleus’ asymmetry should enhance the asymmetry of the atom’s charge.
Questions or comments on this article? E-mail us at feedback@sciencenews.org
CITATIONS
F. Nunes. Why are theorists excited about exotic nuclei? Physics Today. Vol. 74, May 2021, p. 34. doi: 10.1063/PT.3.4748.
L. Neufcourt et al. Quantified limits of the nuclear landscape. Physical Review C. Vol. 101, April 2020, p. 044307. doi: 10.1103/PhysRevC.101.044307.
H. L. Crawford et al. First Spectroscopy of the Near Drip-line Nucleus 40Mg. Physical Review Letters. Vol. 122, February 8, 2019, p. 052501. doi: 10.1103/PhysRevLett.122.052501.
A. B. Balantekin et al. Nuclear Theory and Science of the Facility for Rare Isotope Beams. Modern Physics Letters A. Vol. 29, April 10, 2014, p. 1430010. doi: 10.1142/S0217732314300109.
R. Surman et al. Sensitivity studies for the weak r process: neutron capture rates. AIP Advances. Vol. 4, February 26, 2014, p. 041008. doi: 10.1063/1.4867191.
D.F. Geesaman et al. Physics of a Rare Isotope Accelerator. Annual Review of Nuclear and Particle Science. Vol. 56, April 18, 2006, p. 53. doi: 10.1146/annurev.nucl.55.090704.151604.
Like the interlinked Borromean rings, different facets of nuclear physics are closely entwined, from mysteries of the cosmos to the inner workings of nuclei. The exotic nuclei that FRIB cooks up could also allow physicists to tap into the very bedrock of physics by testing certain fundamental laws of nature. And there’s a practical side to the facility as well. Scientists could collect some of the isotopes FRIB produces for use in medical procedures, for example.
Physicists are ready for surprises. “Every time we build such a facility, new discoveries come and breakthroughs in science come,” Nazarewicz says. Like the 1980s discovery of lithium-11’s Borromean nucleus, scientists may find something totally unexpected.
Icing on the cake
Along with studying atomic nuclei at extremes and exploring nuclear physics of the stars, scientists hope to use FRIB to make progress in two other key areas.

Harvesting useful nuclei
Scientists plan to collect isotopes produced in FRIB for societal applications. In medicine, for example, certain isotopes, such as terbium-149, can be used for radiation treatment or medical imaging.
When this isotope of the rare earth metal terbium decays, it can emit alpha particles (helium nuclei) that can kill cancer cells. Its half-life of 4.1 hours is in a sweet spot: fast enough to have an effect — it doesn’t take hundreds of years to decay — but not so fast that it’s gone within seconds, before it can do its work.

Testing laws of nature
Scientists plan to check certain physics rules, for example, the idea that matter and antimatter behave as mirror images. Certain hypothetical physics effects could cause particles to flout this rule, and that could help explain why there’s more matter than antimatter in the universe.
Effects that could make matter and antimatter behave differently might also cause electric charge in atoms to separate, with slightly more positive charge on one side of the atom and more negative on the other. In most atoms, this separation may be too tiny to measure. But in radium-225, which has a pear-shaped nucleus, the effect would be stronger, as the nucleus’ asymmetry should enhance the asymmetry of the atom’s charge.
Questions or comments on this article? E-mail us at feedback@sciencenews.org
CITATIONS
F. Nunes. Why are theorists excited about exotic nuclei? Physics Today. Vol. 74, May 2021, p. 34. doi: 10.1063/PT.3.4748.
L. Neufcourt et al. Quantified limits of the nuclear landscape. Physical Review C. Vol. 101, April 2020, p. 044307. doi: 10.1103/PhysRevC.101.044307.
H. L. Crawford et al. First Spectroscopy of the Near Drip-line Nucleus 40Mg. Physical Review Letters. Vol. 122, February 8, 2019, p. 052501. doi: 10.1103/PhysRevLett.122.052501.
A. B. Balantekin et al. Nuclear Theory and Science of the Facility for Rare Isotope Beams. Modern Physics Letters A. Vol. 29, April 10, 2014, p. 1430010. doi: 10.1142/S0217732314300109.
R. Surman et al. Sensitivity studies for the weak r process: neutron capture rates. AIP Advances. Vol. 4, February 26, 2014, p. 041008. doi: 10.1063/1.4867191.
D.F. Geesaman et al. Physics of a Rare Isotope Accelerator. Annual Review of Nuclear and Particle Science. Vol. 56, April 18, 2006, p. 53. doi: 10.1146/annurev.nucl.55.090704.151604.
About Emily Conover
Physics writer Emily Conover has a Ph.D. in physics from the University of Chicago. She is a two-time winner of the D.C. Science Writers’ Association Newsbrief award.
No comments:
Post a Comment