It's raining diamonds across the universe, research suggests

It could be raining diamonds on planets throughout the universe, scientists suggested Friday, after using common plastic to recreate the strange precipitation believed to form deep inside Uranus and Neptune.
Scientists had previously theorized that extremely high pressure and temperatures turn hydrogen and carbon into solid diamonds thousands of kilometers below the surface of the ice giants.
Now new research, published in Science Advances, inserted oxygen into the mix, finding that "diamond rain" could be more common than thought.
Ice giants like Neptune and Uranus are thought to be the most common form of planet outside our Solar System, which means diamond rain could be occurring across the universe.
Dominik Kraus, a physicist at Germany's HZDR research lab and one of the study's authors, said that diamond precipitation was quite different to rain on Earth.
Under the surface of the planets is believed to be a "hot, dense liquid", where the diamonds form and slowly sink down to the rocky, potentially Earth-size cores more than 10,000 kilometers (6,200 miles) below, he said.
There fallen diamonds could form vast layers that span "hundreds of kilometers or even more", Kraus told AFP.
While these diamonds might not be shiny and cut like a "a nice gem on a ring", he said they were formed via similar forces as on Earth.
Aiming to replicate the process, the research team found the necessary mix of carbon, hydrogen and oxygen in a readily available source—PET plastic, which is used for everyday food packaging and bottles.
Kraus said that while the researchers used very clean PET plastic, "in principle the experiment should work with Coca-Cola bottles".
The team then turned a high-powered optical laser on the plastic at the SLAC National Accelerator Laboratory in California.
"Very, very short X-ray flashes of incredible brightness" allowed them to watch the process of nanodiamonds—tiny diamonds too small to see with the naked eye—as they formed, Kraus said.
"The oxygen that is present in large amounts on those planets really helps suck away the hydrogen atoms from the carbon, so it's actually easier for those diamonds to form," he added.
New way to make nanodiamonds?
The experiment could point towards a new way to produce nanodiamonds, which have a wide and increasing range of applications including drug delivery, medical censors, non-invasive surgery and quantum electronics.
"The way nanodiamonds are currently made is by taking a bunch of carbon or diamond and blowing it up with explosives," said SLAC scientist and study co-author Benjamin Ofori-Okai.
"Laser production could offer a cleaner and more easily controlled method to produce nanodiamonds," he added.
The diamond rain research remains hypothetical because little is known about Uranus and Neptune, the most distant planets in our Solar System.
Only one spacecraft—NASA's Voyager 2 in the 1980s—has flown past the two ice giants, and the data it sent back is still being used in research.
But a NASA group has outlined a potential new mission to the planets, possibly launching next decade.
"That would be fantastic," Kraus said.
He said he is greatly looking forward to more data—even if it takes a decade or two.'Diamond rain' on giant icy planets could be more common than previously thought
More information: Zhiyu He et al, Diamond formation kinetics in shock-compressed C-H-O samples recorded by small-angle X-ray scattering and X-ray diffraction, Science Advances (2022). DOI: 10.1126/sciadv.abo0617. www.science.org/doi/10.1126/sciadv.abo0617
Journal information: Science Advances
© 2022 AFP
Diamond rain on giant icy planets could be more common than previously thought: Research
An experiment that simulated the conditions on ice giant planets like Neptune and Uranus has helped researchers discover that "diamond rains" may be more common on these planets than previously thought.
By: Tech Desk
Thalassery | September 5, 2022
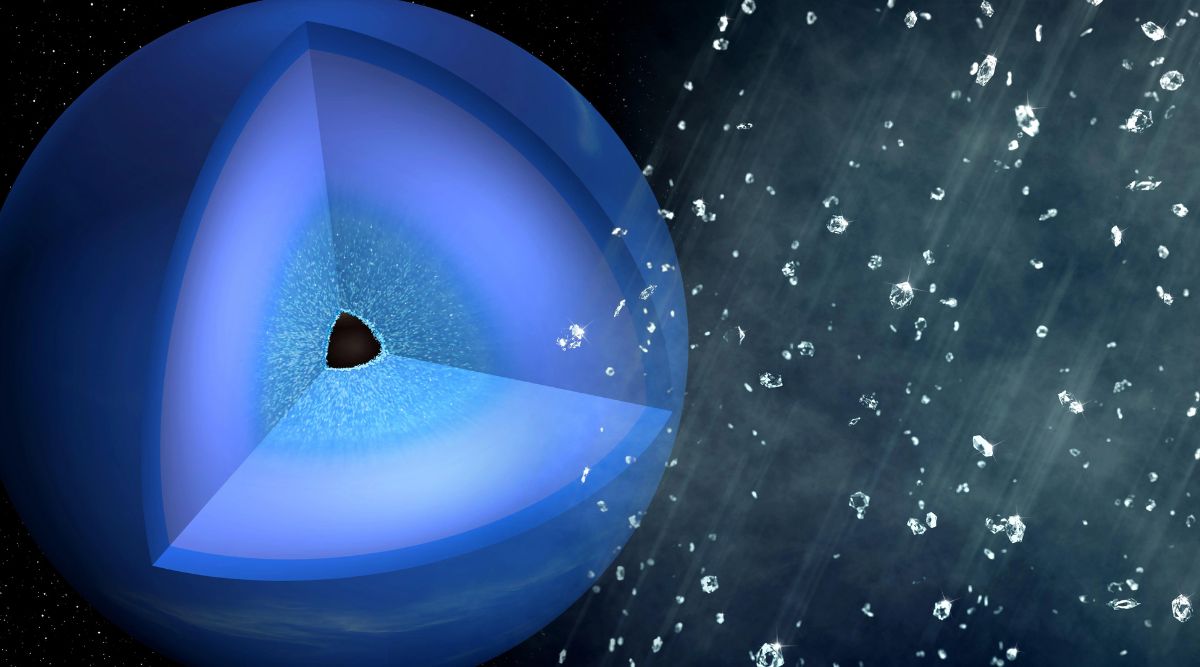
A cutaway that depicts the interior of Neptune with a layer of diamonds surrounding the solid planetary core. (Image credit: Greg Stewart/SLAC National Accelerator Laboratory)
A new study has found that “diamond rain” may be more common on ice giant planets like Neptune and Uranus than previously thought. For the first time, scientists were able to observe diamond rain as it formed with their experiment designed to mimic the extreme temperatures and pressure found on those planets.
Diamond rain forms when hydrogen and carbon found in the interior of these planets are squeezed by the high pressure and form solid diamonds that sink slowly further into the interior. The research has been published in the journal Nature Astronomy.
The researchers simulated the kind of environment found inside these planets by creating shock waves in plastic using an intense laser at the Matter in Extreme Conditions (MEC) instrument at the Stanford National Accelerator Laboratory in Menlo Park, California.
Also Read |Research transforming our knowledge of dementia wins science prize
In the experiment, researchers used PET plastic, often found in food packaging, plastic bottles and containers, to reproduce the composition of these planets. “PET has a good balance between carbon, hydrogen and oxygen to simulate the activity in ice planets,” said Dominik Kraus, a physicist and professor at the University of Rostock, in a press statement.
As they created shockwaves in the plastic using the laser, they observed the atoms of the material rearrange into small diamond regions. They used a method called “small-angle scattering” to measure how fast and large those regions grew. The researchers found that these diamond regions grew up to a size of a few nanometres. With the presence of oxygen in the material, the nanodiamonds were able to grow at lower pressures and temperatures than was observed in previous experiments.
The research team predicts that the diamonds on Neptune and Uranus would become much larger than the nanodiamonds produced in these experiments; maybe even weighing in millions of carats. Over thousands of years, these diamonds could have slowly sunk through the planets’ icy layers to assemble as a thick layer around the solid planetary core.
Also Read |Digging Deep: What the discovery of Ostrich fossils in Himalayas reveals about our climate
This research also opens up the potential to produce nanodiamonds using this laser-driven method. Such nanodiamonds are already included in abrasives and polishing agents and in the future, they can potentially be used for quantum sensors, medical contrast agents and reaction accelerators for renewable energy.
“The way nanodiamonds are currently made is by taking a bunch of carbon or diamond and blowing it up with explosives. This creates nanodiamonds of various sizes and shapes and is hard to control. What we’re seeing in this experiment is a different reactivity of the same species under high temperature and pressure. In some cases, the diamonds seem to be forming faster than others, which suggests that the presence of these other chemicals can speed up this process,” said SLAC scientist and collaborator Benjamin Ofori-Okai, in a press statement.
According to Ofori-Okai, this laser production method could offer a cleaner and more easily controlled method to produce nanodiamonds.
Next, the researchers envision conducting similar experiments using liquid samples containing ethanol, water and ammonia, which is what Uranus and Neptune are mostly made out of. This will help them get even closer to understanding how diamond rain forms on other planet.
© IE Online Media Services Pvt Ltd
First published on: 05-09-2022
No comments:
Post a Comment