Staff Writer | January 13, 2023 |

(Reference image from Raw Pixel.)
A group of scientists has measured the highest toughness ever recorded, of any material, while investigating a metallic alloy made of chromium, cobalt, and nickel (CrCoNi).

In a paper published in the journal Science, the researchers explain that not only is the metal extremely ductile and impressively strong, but its strength and ductility also improve as it gets colder. This runs counter to most other materials in existence.
CrCoNi is a subset of a class of metals called high entropy alloys (HEAs). All the alloys in use today contain a high proportion of one element with lower amounts of additional elements added, but HEAs are made of an equal mix of each constituent element. These balanced atomic recipes appear to bestow some of these materials with an extraordinarily high combination of strength and ductility when stressed, which together make up what is termed “toughness.”
HEAs have been a hot area of research since they were first developed about 20 years ago, but the technology required to push the materials to their limits in extreme tests was not available until recently.
“The toughness of this material near liquid helium temperatures (20 kelvin, -424 Fahrenheit) is as high as 500 megapascals square root meters. In the same units, the toughness of a piece of silicon is one, the aluminum airframe in passenger airplanes is about 35, and the toughness of some of the best steels is around 100. So, 500, it’s a staggering number,” research co-lead Robert Ritchie, a senior faculty scientist at Berkeley Lab, said in a media statement.
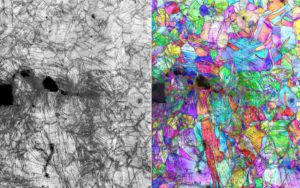
Microscopy-generated images showing the path of a fracture and accompanying crystal structure deformation in the CrCoNi alloy at nanometer scale during stress testing at -424 F.
(Image by Robert Ritchie/Berkeley Lab).
Ritchie and his co-lead Easo George from ORNL began experimenting with CrCoNi and another alloy that also contains manganese and iron (CrMnFeCoNi) nearly a decade ago. They created samples of the alloys then lowered the materials to liquid nitrogen temperatures (around 77 kelvin, or -321 F) and discovered impressive strength and toughness.
Given those results, they immediately wanted to follow up their work with tests at liquid helium temperature ranges, but finding facilities that would enable stress testing samples in such a cold environment, and recruiting team members with the analytical tools and experience needed to analyze what happens in the material at an atomic level took the next 10 years.
The pair explained that many solid substances, including metals, exist in a crystalline form characterized by a repeating 3D atomic pattern, called a unit cell, that makes up a larger structure called a lattice. The material’s strength and toughness, or lack thereof, come from the physical properties of the lattice. No crystal is perfect, so the unit cells in a material will inevitably contain “defects,” a prominent example being dislocations – boundaries where undeformed lattice meets up with deformed lattice.
When force is applied to the material – think, for example, of bending a metal spoon – the shape change is accomplished by the movement of dislocations through the lattice. The easier it is for the dislocations to move, the softer the material is. But if the movement of the dislocations is blocked by obstacles in the form of lattice irregularities, then more force is required to move the atoms within the dislocation, and the material becomes stronger. On the flip side, obstacles usually make the material more brittle – prone to cracking.
Ritchie and his co-lead Easo George from ORNL began experimenting with CrCoNi and another alloy that also contains manganese and iron (CrMnFeCoNi) nearly a decade ago. They created samples of the alloys then lowered the materials to liquid nitrogen temperatures (around 77 kelvin, or -321 F) and discovered impressive strength and toughness.
Given those results, they immediately wanted to follow up their work with tests at liquid helium temperature ranges, but finding facilities that would enable stress testing samples in such a cold environment, and recruiting team members with the analytical tools and experience needed to analyze what happens in the material at an atomic level took the next 10 years.
The pair explained that many solid substances, including metals, exist in a crystalline form characterized by a repeating 3D atomic pattern, called a unit cell, that makes up a larger structure called a lattice. The material’s strength and toughness, or lack thereof, come from the physical properties of the lattice. No crystal is perfect, so the unit cells in a material will inevitably contain “defects,” a prominent example being dislocations – boundaries where undeformed lattice meets up with deformed lattice.
When force is applied to the material – think, for example, of bending a metal spoon – the shape change is accomplished by the movement of dislocations through the lattice. The easier it is for the dislocations to move, the softer the material is. But if the movement of the dislocations is blocked by obstacles in the form of lattice irregularities, then more force is required to move the atoms within the dislocation, and the material becomes stronger. On the flip side, obstacles usually make the material more brittle – prone to cracking.
Peeking inside CrCoNi
Using neutron diffraction, electron backscatter diffraction, and transmission electron microscopy, Ritchie, George, and their colleagues at Berkeley Lab, the University of Bristol, Rutherford Appleton Laboratory, and the University of New South Wales examined the lattice structures of CrCoNi samples that had been fractured at room temperature and 20 K.
The images and atomic maps generated from these techniques revealed that the alloy’s toughness is due to a trio of dislocation obstacles that come into effect in a particular order when force is applied to the material.
First, moving dislocations cause areas of the crystal to slide away from other areas that are on parallel planes. This movement displaces layers of unit cells so that their pattern no longer matches up in the direction perpendicular to the slipping movement, creating a type of obstacle.
Further force on the metal creates a phenomenon called nanotwinning, wherein areas of the lattice form a mirrored symmetry with a boundary in between. Finally, if forces continue to act on the metal, the energy being put into the system changes the arrangement of the unit cells themselves, with the CrCoNi atoms switching from a face-centred cubic crystal to another arrangement known as hexagonal close packing.
This sequence of atomic interactions ensures that the metal keeps flowing, but also keeps meeting new resistance from obstacles far past the point that most materials snap from the strain.
“So as you are pulling it, the first mechanism starts and then the second one starts, and then the third one starts, and then the fourth,” explained Ritchie. “Now, a lot of people will say, well, we’ve seen nanotwinning in regular materials, we’ve seen slip in regular materials. That’s true. There’s nothing new about that, but it’s the fact they all occur in this magical sequence that gives us these really tremendous properties.”
The team’s new findings, taken with other recent work on HEAs, may force the materials science community to reconsider long-held notions about how physical characteristics give rise to performance.
“It’s amusing because metallurgists say that the structure of a material defines its properties, but the structure of the NiCoCr is the simplest you can imagine – it’s just grains,” said Ritchie. “However, when you deform it, the structure becomes very complicated, and this shift helps explain its exceptional resistance to fracture,” co-author Andrew Minor said.
New products…but not just yet
George foresees the new material being used in situations where environmental extremes could destroy standard metallic alloys, such as in the frigid temperatures of deep space.
He and his team at the Oak Ridge National Laboratory are also investigating how alloys made of more abundant and less expensive elements could be coaxed into having similar properties.
Though the progress is exciting, Ritchie warns that real-world use could still be a ways off, for good reason.
“When you are flying on an airplane, would you like to know that what saves you from falling 40,000 feet is an airframe alloy that was only developed a few months ago? Or would you want the materials to be mature and well-understood? That’s why structural materials can take many years, even decades, to get into real use.”
No comments:
Post a Comment