The Conversation
March 27, 2023

Shutterstock
Since it was founded in 2016, Elon Musk’s brain-computer interface (BCI) company Neuralink has had its moments in biotech news.
Whether it was the time Musk promised his “link” would let people communicate telepathically, or when the whole company was under investigation for potentially violating the Animal Welfare Act, the hype around Neuralink means it’s often the first mental reference people have for BCI technology.
But BCIs have been kicking around for much longer than you’d expect. Musk’s is just one in a growing list of companies dedicated to advancing this technology. Let’s take a look back at some BCI milestones over the past decades, and forward to where they might lead us.
An expanding sector
Brain-computer interfaces are devices that connect the brain with a computer to allow the user to complete some kind of action using their brain signals.
Many high-profile companies entered the BCI field in the 2010s, backed by millions of dollars in investment. Founded in 2016, the American company Kernel began by researching implantable devices, before switching to focus on non-invasive techniques that don’t require surgery.
Even Facebook gave BCIs a go, with an ambitious plan to create a headset that would let users type 100 words per minute. But it stopped this research in 2021 to focus on other types of human-computer interfaces.
First contact
Developed in the 1970s, the earliest BCIs were relatively straightforward, used on cats and other animals to develop communication pathways. The first device implanted in a human was developed by Jonathan Wolpaw in 1991, and allowed its user to control a cursor with their brain signals.
Advances in machine learning through the years paved the way for more sophisticated BCIs. These could control complex devices, including robotic limbs, wheelchairs and exoskeletons. We’ve also seen devices get progressively smaller and easier to use thanks to wireless connectivity.
Like many newer BCI devices, Neuralink has yet to receive approval for clinical trials of its invasive implant. Its latest application to the US Food and Drug Administration was rejected.
There are, however, three notable groups conducting clinical trials that are worth keeping an eye on.
1. BrainGate
Founded in 1998 in Massachusetts, the BrainGate system has been around since the late 1990s. This makes it one of the oldest advanced BCI implant systems. Its device is placed in the brain using microneedles, similar to the technology Neuralink uses.
BrainGate’s devices are probably the most advanced when it comes to BCI functionality. One of its wired devices offers a typing speed of 90 characters per minute, or 1.5 characters per second. A study published in January released results from data collected over 17 years from 14 participants.
During this time there were 68 instances of “adverse events” including infection, seizures, surgical complications, irritation around the implant, and brain damage. However, the most common event was irritation. Only six of the 68 incidents were considered “serious”.
Apart from communication applications, BrainGate has also achieved robotic control for self-feeding.
2. UMC Utrecht
The University Medical Centre in Utrecht, Netherlands, was the first to achieve fully wireless implanted BCI technology that patients could take home.
Its device uses electrocorticography-based BCI (ECoG). Electrodes in the form of metal discs are placed directly on the surface of the brain to receive signals. They connect wirelessly to a receiver, which in turn connects to a computer.
Participants in a clinical trial that ran from 2020 to 2022 were able to take the device home and use it every day for about a year. It allowed them to control a computer screen and type at a speed of two characters per minute.
While this typing speed is slow, future versions with more electrodes are expected to perform better.
3. Synchron (originally SmartStent)
Synchron was founded in 2016 in Melbourne, Australia. In 2019, it became the first company to be approved for clinical trials in Australia. Then in 2020 it became the first company to receive FDA approval to run clinical trials using a permanently implanted BCI – and finally did this with a US patient this year.
Synchron’s approach is to bypass full brain surgery by using blood vessels to implant electrodes in the brain. This minimally invasive approach is similar to other stenting procedures routinely performed in clinics.
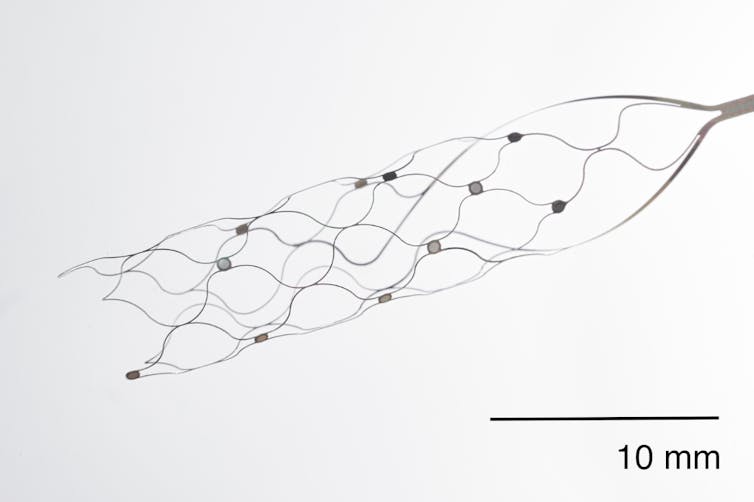
Synchron’s very small ‘stentrode’ can be implanted with a minimally invasive procedure. Synchron
Synchron’s device is placed in the brain near the area that controls movement, and a wireless transmitter is placed in the chest. This transmitter then conveys brain signals to a computer.
Initial clinical results have shown no adverse effects and a functionality of 14 characters per minute using both the BCI and eye tracking. Results were not reported for BCI use alone.
Although its device efficiency could be improved, Synchron’s approach means it leads the way in achieving a low barrier for entry. By avoiding the need for full brain surgery, it’s helping to bring BCI implantation closer to being a day procedure.
The benefits must outweigh the risks
The history of BCIs reveals the immense challenges involved in developing this technology. These are compounded by the fact that experts still don’t fully understand the links between our neural circuitry and thoughts.
It’s also unclear which BCI features consumers will prioritize moving forward, or what they’d be willing to sign up for. Not everyone will happily opt for an invasive brain procedure – yet the systems that don’t require this collect “noisy” data that aren’t as efficient.

Electroencephalogram-based (EEG) BCIs don’t require surgery, but being less invasive means they’re also less effective. Shutterstock
Answers will emerge as more devices gain approval for clinical trials and research is published on the results.
Importantly, developers of these technologies must not rush through trials. They have a responsibility to be transparent about the safety and efficacy of their devices, and to report on them openly so consumers can make informed decisions.

Sam John, Senior Lecturer in Neural Engineering, The University of Melbourne
This article is republished from The Conversation under a Creative Commons license. Read the original article.
No comments:
Post a Comment