Virus-like transposons cross the species barrier, study shows
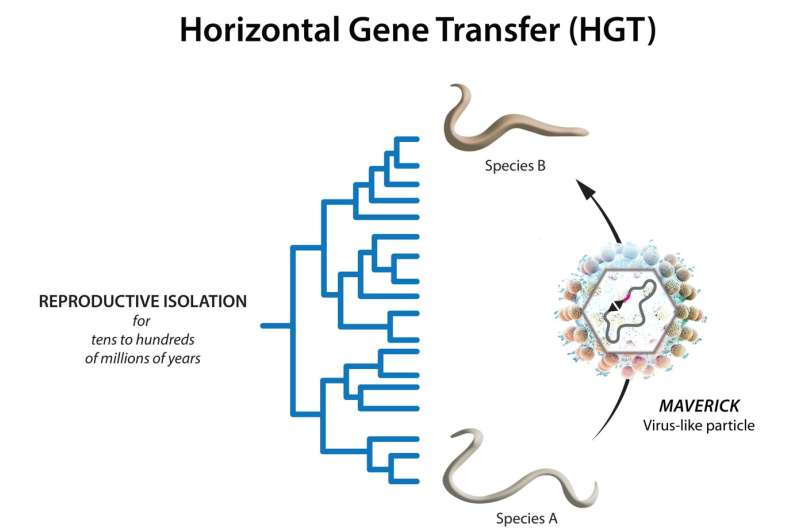
Scientists have known for decades that genes can be transferred from one species to another, both in animals and plants. However, the mechanism of how such an unlikely event occurs remained unknown. Now, researchers from Alejandro Burga's lab at the Institute of Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences identify a vector of horizontal gene transfer (HGT) in worms. The findings, published in Science, could lead to the discovery of further vectors of HGT in eukaryotes and might find applications in pathogen control.
Fish living in the Arctic and Antarctic oceans have evolved ingenious strategies to prevent their blood and tissues from freezing in the inhospitable polar waters. One such adaptive strategy is the evolution of genes that produce antifreeze proteins. However, over a decade ago, scientists were astounded to discover that herrings and smelts—two completely different species—have the exact same antifreeze protein encoded in their genomes, indicating a gene transfer between them.
Examples such as this raise the question: how can genes "jump" between completely different species? This rare phenomenon, known as HGT, has puzzled evolutionary biologists for a long time. And despite new instances of HGT being discovered across all branches of life over the years, the mechanisms responsible for these transfers have largely remained unknown.
Now, scientists from Alejandro Burga's group at IMBA not only catch an HGT event in the animal kingdom red-handed, but they also identify one of its long-sought vectors. By means of genetic detective work, Burga and his team showed an event of HGT between two reproductively isolated worm species that are genetically as different from each other as humans are from fish. More importantly, they could identify what caused it: a family of virus-like transposons called Mavericks.
Nailing down a culprit: Mavericks as vectors of HGT
"Mavericks were already known as a class of transposons, but our work links them to HGT for the first time," says IMBA group leader Alejandro Burga, the study's corresponding author. "We knew that HGT did take place between animal species, but we had no idea how. This is the first time that we could definitively nail down a culprit," adds co-first author Sonya Widen, a postdoctoral fellow in the Burga lab.
When Mavericks were discovered in the mid-2000s, they were initially thought to be large transposons, selfish genetic elements that jump and self-propagate in the genome at the expense of their host. Mavericks were quickly reported in most branches of eukaryotes, including humans, thus establishing that they originated a long time ago.
Transposons and viruses, nature's melting pot?
Soon, evidence that Mavericks contained genes encoding viral elements, such as a capsid and a DNA polymerase, started to surface. "The evolution of transposons and viruses is tightly intertwined," says Burga. However, the capsid and the DNA polymerase are not enough to allow a transposon to jump from its host's genome and infect the cells of a completely different host.
Now, the IMBA researchers found the missing link: Mavericks in worm genomes have acquired a so-called fusogen protein, a transmembrane protein that mediates membrane fusion between different cells. By acquiring a fusogen, the authors hypothesize that worm Mavericks became capable of forming virus-like particles that can fuse with another organism's cell membranes and infect them.
"To our knowledge, no fusogen has been reported in Mavericks before. Thus, we think that worm Mavericks might have picked up their sequence from a virus," says Widen. "Transposons and viruses can be thought of as nature's melting pot. Their union can have unpredictable repercussions and lead to genomic innovation," says Burga.
Demonstrating the significance of HGT in worms
In the study, the IMBA team led by Alejandro Burga and co-first authors Sonya Widen and Israel Campo Bes, a former master's student in the Burga lab, came across HGT "totally by chance," as Widen says. In fact, the team was studying the evolutionary origin of a selfish element in the nematode Caenorhabditis briggsae. Doing some detective work, they were able to trace the sequence of this selfish gene back to another nematode, C. plicata, which carried an almost identical copy.
This finding is surprising because C. briggsae and C. plicata are two reproductively isolated species. "Their genomes are as divergent as those of humans and fish, and yet they both have an almost identical gene that clearly shows features of an evolutionarily recent HGT event," says Campo Bes.
"By carefully looking at the genome of C. plicata, we found that the ancestral sequence that gave rise to the selfish gene in C. briggsae was embedded inside a Maverick in C. plicata. The fact that this newly introduced gene subsequently evolved into a novel selfish gene in C. briggsae demonstrates the impact of HGT on genome evolution," Widen explains. The IMBA team then went on to show that Mavericks are responsible for dozens of independent HGT transfer events between worm species belonging to different genera and found all around the globe.
Agricultural and medical relevance
The IMBA scientists argue that the union between transposons and viruses is a key factor in mediating HGT. While still finding it hard to believe their success, they recognize the impact that their findings could have on lifting the mysteries of HGT. "I was convinced that we were looking at a case of HGT when we first saw these results in the lab, but I was also sure that we would never find out how it happened. Yet, the stars aligned," says Burga, who also predicts that Mavericks and similar virus-like transposable elements could mediate HGT in vertebrates and other eukaryotes.
Finally, the team foresees possible applications both in the lab and as pest control measures against parasitic worm species. "If Maverick-mediated HGT is shown to be broadly applicable to any nematode species, it has the potential to become an invaluable resource. Beyond strict lab and research applications such as the genetic manipulation of non-model nematodes, such a resource could allow us, in the future, to genetically modify parasitic nematode species that might be of agricultural or medical relevance," concludes Burga.
More information: Sonya A. Widen et al, Virus-like transposons cross the species barrier and drive the evolution of genetic incompatibilities, Science (2023). DOI: 10.1126/science.ade0705. www.science.org/doi/10.1126/science.ade0705
Journal information: Science
Provided by IMBA- Institute of Molecular Biotechnology of the Austrian Academy of Sciences
No comments:
Post a Comment