Illinois Tech engineer spearheads research leading to groundbreaking green propane production method
Mohammad Asadi partners with SHV Energy to distribute electrolyzer device that can convert carbon dioxide into propane in a way that is economically viable and scalable
IMAGE: ILLUSTRATION OF ELECTROLYZER, WHICH USES A NOVEL CATALYST TO CONVERT CARBON DIOXIDE INTO PROPANE. view more
CREDIT: ILLINOIS INSTITUTE OF TECHNOLOGY
CHICAGO—August 18, 2023—A paper recently published in Nature Energy based on pioneering research done at Illinois Institute of Technology reveals a promising breakthrough in green energy: an electrolyzer device capable of converting carbon dioxide into propane in a manner that is both scalable and economically viable.
As the United States races toward its target of net-zero greenhouse gas emissions by 2050, innovative methods to reduce the significant carbon dioxide emissions from electric power and industrial sectors are critical. Mohammad Asadi, assistant professor of chemical engineering at Illinois Tech, spearheaded this groundbreaking research.
“Making renewable chemical manufacturing is really important,” says Asadi. “It’s the best way to close the carbon cycle without losing the chemicals we currently use daily.”
What sets Asadi’s electrolyzer apart is its unique catalytic system. It uses inexpensive, readily available materials to produce tri-carbon molecules—fundamental building blocks for fuels like propane, which is used for purposes ranging from home heating to aviation.
To ensure a deep understanding of the catalyst’s operations, the team employed a combination of experimental and computational methods. This rigorous approach illuminated the crucial elements influencing the catalyst’s reaction activity, selectivity, and stability.
A distinctive feature of this technology, lending to its commercial viability, is the implementation of a flow electrolyzer. This design permits continuous propane production, sidestepping the pitfalls of the more conventional batch processing methods.
“Designing and engineering this laboratory-scale flow electrolyzer prototype has demonstrated Illinois Tech’s commitment to creating innovative technologies. Optimizing and scaling up this prototype will be an important step toward producing a sustainable, economically viable, and energy-efficient carbon capture and utilization process,” says Advanced Research Projects Agency-Energy Program Director Jack Lewnard.
This innovation is not Asadi’s first venture into sustainable energy. He previously adapted a version of this catalyst to produce ethanol by harnessing carbon dioxide from industrial waste gas. Recognizing the potential of the green propane technology, Asadi has collaborated with global propane distributor SHV Energy to further scale and disseminate the system.
“This is an exciting development which opens up a new e-fuel pathway to on-purpose propane production for the benefit of global users of this essential fuel,” says Keith Simons, head of research and development for sustainable fuels at SHV Energy.
Illinois Tech Duchossois Leadership Professor and Professor of Physics Carlo Segre, University of Pennsylvania Professor of Materials Science and Engineering Andrew Rappe, and University of Illinois Chicago Professor Reza Shahbazian-Yassar contributed to this work. Mohammadreza Esmaeilirad (Ph.D. CHE ’22) was a lead author on the paper.
Disclaimer: “Research reported in this publication was supported by the National Science Foundation under Award Number 2135173, the Advanced Research Projects Agency-Energy under Award Number DE-AR0001581, and SHV Energy. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation, the Advanced Research Projects Agency-Energy, or SHV Energy.”
Mohammad Asadi, “Imidazolium Functionalized Transition Metal Phosphide Catalysts for Electrochemical Carbon Dioxide Conversion to Ethanol,” National Science Foundation; Award Number 2135173
Mohammad Asadi, “Direct Conversion of Flue Gas to Value-Added Chemicals Using a Carbon-Neutral Process,” Advanced Research Projects Agency-Energy; Award Number DE-AR0001581
JOURNAL
Nature Energy
METHOD OF RESEARCH
Experimental study
SUBJECT OF RESEARCH
Not applicable
ARTICLE TITLE
Imidazolium-functionalized Mo3P nanoparticles with an ionomer coating for electrocatalytic reduction of CO2 to propane
ARTICLE PUBLICATION DATE
17-Aug-2023
Researchers unveil a new, economical approach for producing green hydrogen
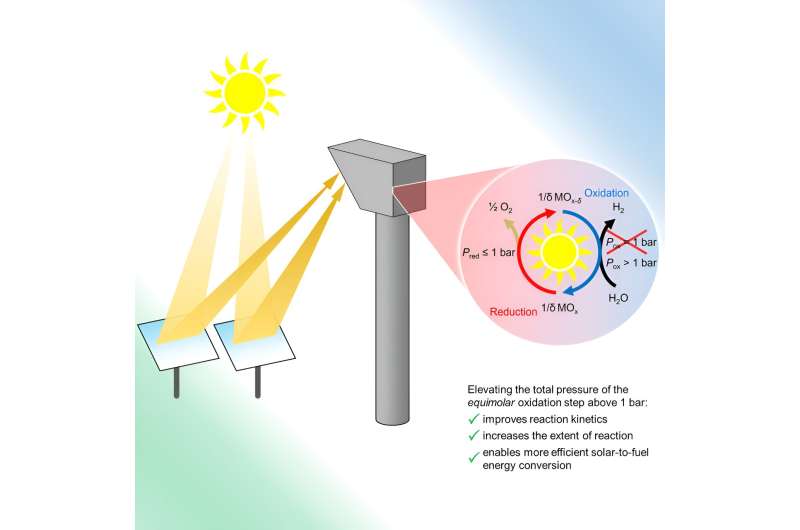
Researchers at the University of Colorado have developed a new and efficient way to produce green hydrogen or green syngas, a precursor to liquid fuels. The findings could open the door for more sustainable energy use in industries like transportation, steelmaking and ammonia production.
The new study, published Aug. 16 in the journal Joule, focuses on the production of hydrogen or syngas, a mixture of hydrogen and carbon monoxide that can be converted into fuels like gasoline, diesel and kerosene. The CU Boulder team lays the groundwork for what could be the first commercially viable method for producing this fuel, entirely using solar energy. That might help engineers to generate syngas in a more sustainable way.
The group was led by Al Weimer, professor in the Department of Chemical and Biological Engineering.
"The way I like to think about it is some day when you go to the pump you'll have, for example, unleaded, super unleaded and ethanol options, and then an additional option being solar fuel, where the fuel is derived from sunlight, water and carbon dioxide," said Kent Warren, one of two lead authors of the new study and a research associate in Chemical and Biological Engineering. "Our hope is that it will be cost-competitive to the fuels sourced from the ground."
Traditionally, engineers produce hydrogen gas through electrolysis, or using electricity to split molecules of water into hydrogen and oxygen gas. The team's "thermochemical" approach, in contrast, uses heat generated by solar rays to complete those same chemical reactions. The methods can also split molecules of carbon dioxide pulled from the atmosphere to produce carbon monoxide.
Scientists had previously shown that such an approach to making hydrogen and carbon monoxide was possible, but might not be efficient enough to produce syngas in a commercially viable manner.
In the new study, the researchers demonstrated that they can conduct these reactions at elevated pressures, in part by employing iron-aluminate materials, which are relatively inexpensive and abundant in the Earth. Those higher pressures allowed the team to more than double its production of hydrogen.
More information: Justin T. Tran et al, Pressure-enhanced performance of metal oxides for thermochemical water and carbon dioxide splitting, Joule (2023). DOI: 10.1016/j.joule.2023.07.016

No comments:
Post a Comment