POSTMODERN ALCHEMY
Gold nanoclusters can improve electrochemical water splitting to produce hydrogen
As energy demand continues to rise, research into new, efficient renewable and clean energy sources is an urgent priority. Currently, renewable energy sources like solar, wind, tide, and geothermal make up less than 40% of the current energy demand. Increasing this percentage and reducing the amount of fossil fuels used will require other, more efficient renewable and clean energy sources.
Hydrogen is a promising alternative, but it is currently produced using steam reforming, which is inefficient and produces CO2 emissions. Electrochemical water splitting, also called water electrolysis, can take advantages of the electricity generated from renewable sources, is a potential efficient solution to produce hydrogen.
Water splitting requires a reaction called hydrogen evolution reaction (HER), but the nanocatalysts involved in this HER do not have uniform size, composition, structure, or chemical coordination environment to improve the efficiency and promote the reaction mechanistic understanding. The solution to this problem may lie in atomically precise gold nanoclusters.
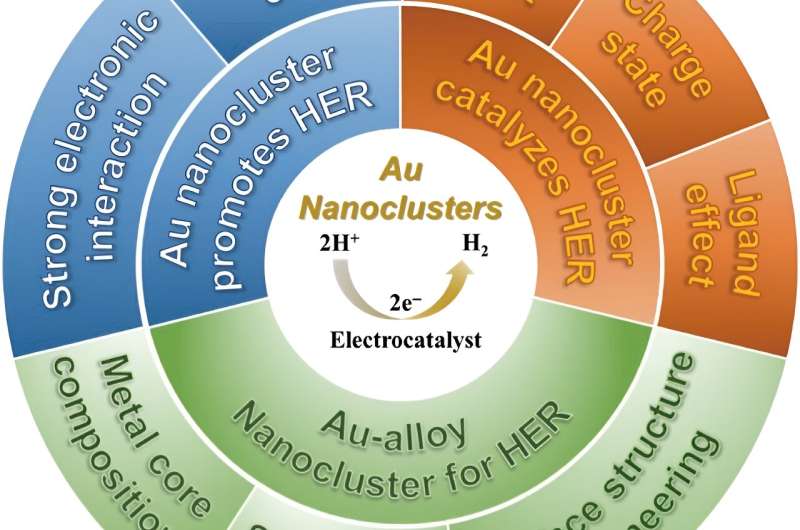
In a literature review published in Polyoxometalates on August 19, the researchers summarize existing work that studies how gold nanoclusters can improve catalytic performance and promote HER.
"It is extremely difficult to achieve a model catalyst with absolute uniform size, definite geometric configuration, and a well-defined local chemical environment at the anatomical level to establish the unambiguous atomical-level structure-performance relationship. Atomically precise gold nanoclusters can potentially resolve those issues," said Zhenghua Tang, a researcher at the New Energy Research Institute at the South China University of Technology in Guangzhou, China. "Specifically, gold nanoclusters have demonstrated extraordinary catalytic properties in various organic reactions and electrocatalytic reactions."
Gold nanocluster is uniquely suited to be a catalyst for HER for several reasons. Unlike other nanocatalysts, gold nanocluster has a precise nanostructure. This precise structure means that all gold nanoclusters are uniform in size, composition, morphology, and chemical environment.
It is also helpful for identifying the active sites for HER catalysis. The rich chemical reactivities of gold nanoclusters allow for both metal core tailoring and surface ligand engineering. Metal core tailoring is when another metal is introduced to the gold nanocluster, which forms a gold-alloy cluster. Introducing another metal can endow novel catalytic capabilities and cut down the cost. In surface ligand engineering, the surface chemical environment can be fine-tuned to expose more active sites or change the structure of the nanocluster.
Finally, gold nanoclusters have other structural merits, such as the size is ultrasmall, which meets the principle of "small is precious" in catalysis field; the morphology can be tuned and manipulated; robust stability with intact structure preserved in various reaction under mild conditions.
"The cases presented in this review clearly show that exceptional HER catalytic properties are often displayed because of the distinct advantages of gold nanoclusters compared to gold nanoparticles. However, challenges are certainly present in employing gold nanoclusters for HER catalysis," said Tang.
Some of the common challenges associated with gold nanoclusters are finding a solution to the amount of gold that would be required to scale the use of these catalysts, problems with how the nanocatalysts perform in harsh conditions, and inaccurate theoretical modeling.
Looking ahead, researchers are planning what the next steps in nanocatalyst research should be. Suggested avenues include testing the applicability of the gold cluster-based composite for other reactions coupled with HER and improving the electrical conductivity of the cluster-based composite catalyst.
"Due to the rapid development of synthetic techniques and catalysis science, we anticipate more research efforts will be dedicated to using atomically precise metal nanoclusters as model catalysts for various electrocatalytic reactions and beyond," said Tang.
More information: Xin Zhu et al, Atomically precise Au nanoclusters for electrochemical hydrogen evolution catalysis: Progress and perspectives, Polyoxometalates (2023). DOI: 10.26599/POM.2023.9140031
Provided by Tsinghua University Press
No comments:
Post a Comment