ALCHEMY
Tiny sample from corner of Mona Lisa reveals toxic secret hidden inside paintingVishwam Sankaran
Mon, 16 October 2023

Samples taken from Leonardo da Vinci’s iconic paintings Mona Lisa and the Last Supper suggest the renowned Renaissance artist played around with chemicals, causing a rare toxic compound to form in his artworks.
He experimented with the compound lead (II) oxide, causing the formation of the toxic lead-compound plumbonacrite in a layer beneath the iconic Mona Lisa painting, new research published last week in the Journal of the American Chemical Society suggested.
Previous research has shown that many paintings from the early 1500s, including the Mona Lisa, were painted on wooden panels that required a thick, “ground layer” of paint to be laid down before artwork was added.
While other artists of the time typically used gesso – a compound derived from plaster of Paris – the Italian polymath widely experimented, say scientists, including those from CNRS in France.
They say he began some of his artworks by laying down thick layers of lead white pigment and by infusing his oil with lead(II) oxide – an orange pigment that conferred specific drying properties to the paint above.
He also used a similar technique on the wall underneath the Last Supper, which researchers say is a departure from the traditional, fresco mural painting technique used at the time.
In the latest study, scientists sought to apply updated and high-resolution analytical techniques to small samples from these two paintings.
They assessed a tiny “microsample” that was previously obtained from a hidden corner of the “Mona Lisa,” as well as 17 such small samples taken from across the surface of the “Last Supper.”
The study found that the ground layers of these artworks not only contained oil and lead white but also a much rarer lead compound: plumbonacrite [Pb5(CO3)O(OH)2].
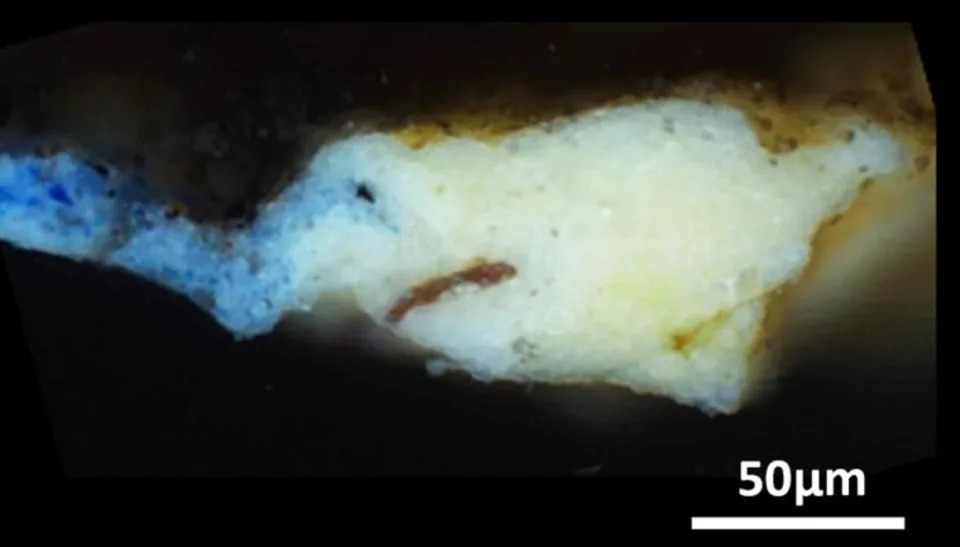
This tiny fleck of paint, taken from the “Mona Lisa” is revealing insights into previously unknown steps of the artists’ process (Adapted from the Journal of the American Chemical Society, 2023)
While this material had not been previously detected in Italian Renaissance paintings, researchers say it has been found in later paintings by Rembrandt in the 1600s.
Although artists are known to have added lead oxides to pigments to help them dry, this technique has not been proved experimentally for paintings from da Vinci’s time.
During the legendary polymath’s time, the only evidence scientists have found of PbO was in reference to skin and hair remedies, even though it is now known to be toxic.
The Mona Lisa Reveals a Toxic Secret Hidden Inside The Painting
Story by David Nield •5h

Mona Lisa Cropped© Provided by ScienceAlert
Leonardo da Vinci is well known for having used less conventional painting methods and substances in his work, and we're still making new discoveries about them – the latest being a mix of toxic pigments underlying the brushwork on the Mona Lisa.
Researchers from France and the UK looked at a tiny microsample taken from a hidden corner of the Mona Lisa, deploying a variety of X-ray diffraction and infrared spectroscopy imaging techniques to identify the substances used.
The team found not only oil and lead white – as expected – but also the rare compound plumbonacrite (Pb5(CO3)3O(OH)2). Plumbonacrite is formed when oil and lead(II) oxide (or PbO) react together, suggesting the latter compound was used by da Vinci.

The microsample analyzed by the team. (Gonzalez et al., Journal of the American Chemical Society , 2023)© Provided by ScienceAlert
"Leonardo probably endeavored to prepare a thick paint suitable for covering the wooden panel of the Mona Lisa by treating the oil with a high load of lead(II) oxide, PbO," write the researchers in their published paper.
The same PbO compound was found in several microsamples taken from the surface of The Last Supper, another famous painting from da Vinci. However, the only references to PbO in the Italian artist's writings were related to skin and hair remedies.
Even though it's not included in his writings, it nevertheless seems that da Vinci made use of this lead(II) oxide as a ground layer. It's something that's been hypothesized about before, but now we have more direct evidence of it.
It's thought that the lead(II) oxide power would've been heated and dissolved in linseed or nut oil by da Vinci, producing a mixture that's thicker and faster drying than traditional oil paints – a recipe that then went on to be used by other artists.
The same plumbonacrite substance has also been discovered in The Night Watch painting by Rembrandt, created in 1642 – almost a century and a half after the Mona Lisa. That suggests the Dutch master used a similar technique to da Vinci.
This discovery is another example of how modern-day analysis techniques are opening up new findings about historical artifacts. Advanced 3D rendering has previously been used to help study another da Vinci painting, Salvator Mundi.
It's also testament to the constant inventiveness of Leonardo da Vinci, a man who achieved greatness not just in his painting, but also in many other fields – including mathematics, chemistry, and engineering.
"He was someone who loved to experiment, and each of his paintings is completely different technically," chemist Victor Gonzalez, from the Institut de Recherche de Chimie Paris in France, told the Associated Press.
The research has been published in the Journal of the American Chemical Society.
No comments:
Post a Comment