BY TIM WOGAN
30 JANUARY 2024
CHEMISTRY WORLD
Red mud – a strongly alkaline, potentially toxic slurry generated in huge quantities by aluminium production – could be transformed into a safe, useful building material using a new hydrogen plasma-based reduction process. The method, developed by researchers in Germany, offers a green source of iron that could be used in steelmaking, and it could also be used to extract other valuable rare-earth metals.
Aluminium is one of the most in-demand materials on Earth, used in everything from civil engineering structures and lightweight vehicle bodies to food packaging. To produce metallic aluminium, alumina (aluminium oxide) must first be purified from the sedimentary rock bauxite. This is usually accomplished using the Bayer process, of which the first step involves heating with sodium hydroxide to dissolve the alumina, forming sodium aluminate. Red mud is the byproduct, and the backlog of around 4.5 billion tonnes grows by 180 million tonnes each year. ‘In some countries it’s absolutely shocking – they just pour it straight into the jungle,’ says Dierk Raabe at the Max Planck Institute for Iron Research in Düsseldorf. ‘In Europe we put it into huge basins.’ In 2010, the collapse of one of these basins led to an infamous disaster in Hungary that caused 10 deaths and cost nearly £100 million to clean up.
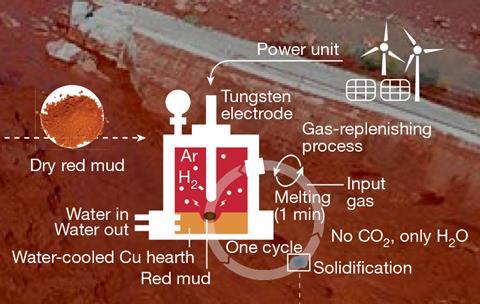
Source: © Matic Jovičević-Klug et al 2024
A scheme showing how the new process extracts solid metal from red mud
There is practically no industry recycling of the red mud, notes Raabe. Efforts to develop such processes involve sintering the material into ceramics that can be used in construction. But at present only 3% of the material is recycled – something Raabe is keen to change. ‘In principle, we say you want to extract all the metal value of it,’ he says.
The exact composition of red mud varies depending on the bauxite source: it often contains traces of valuable rare-earth metals such as yttrium and scandium and potentially toxic heavy metals such as cadmium and chromium as well as unreacted alumina. One constant, however – and the source of the red colour – is the most widely used metal in the world: iron. The production of iron is also a severe sustainability problem because producing it from iron ore involves the use of coke as a reductant, which releases huge amounts of carbon dioxide.
Related stories
CHEMISTRY WORLD
Red mud – a strongly alkaline, potentially toxic slurry generated in huge quantities by aluminium production – could be transformed into a safe, useful building material using a new hydrogen plasma-based reduction process. The method, developed by researchers in Germany, offers a green source of iron that could be used in steelmaking, and it could also be used to extract other valuable rare-earth metals.
Aluminium is one of the most in-demand materials on Earth, used in everything from civil engineering structures and lightweight vehicle bodies to food packaging. To produce metallic aluminium, alumina (aluminium oxide) must first be purified from the sedimentary rock bauxite. This is usually accomplished using the Bayer process, of which the first step involves heating with sodium hydroxide to dissolve the alumina, forming sodium aluminate. Red mud is the byproduct, and the backlog of around 4.5 billion tonnes grows by 180 million tonnes each year. ‘In some countries it’s absolutely shocking – they just pour it straight into the jungle,’ says Dierk Raabe at the Max Planck Institute for Iron Research in Düsseldorf. ‘In Europe we put it into huge basins.’ In 2010, the collapse of one of these basins led to an infamous disaster in Hungary that caused 10 deaths and cost nearly £100 million to clean up.

Source: © Matic Jovičević-Klug et al 2024
A scheme showing how the new process extracts solid metal from red mud
There is practically no industry recycling of the red mud, notes Raabe. Efforts to develop such processes involve sintering the material into ceramics that can be used in construction. But at present only 3% of the material is recycled – something Raabe is keen to change. ‘In principle, we say you want to extract all the metal value of it,’ he says.
The exact composition of red mud varies depending on the bauxite source: it often contains traces of valuable rare-earth metals such as yttrium and scandium and potentially toxic heavy metals such as cadmium and chromium as well as unreacted alumina. One constant, however – and the source of the red colour – is the most widely used metal in the world: iron. The production of iron is also a severe sustainability problem because producing it from iron ore involves the use of coke as a reductant, which releases huge amounts of carbon dioxide.
Related stories

Waste gases from steelmaking repurposed to prepare pharmaceuticals

Hungary’s rivers in recovery after red mud disaster
A carbon-free process that reduced red mud to release the iron would therefore offer a tantalising bipartite solution, and is something Raabe’s team aims to achieve using plasma chemistry. The researchers placed red mud samples in an electric arc furnace before injecting a mixture of hydrogen and argon. They then used a 200A discharge to simultaneously melt the sample and ionise the hydrogen, forcing it to react with the iron. The resulting metallic iron, which chemically partitioned from the oxide byproducts, was purer than from a typical blast furnace and could be used directly for steelmaking. After the researchers repeated the procedure six times, they extracted 2.6g of metallic iron from 15g of red mud – around 98% of the theoretical limit.
The ‘real breakthrough’, explains Raabe, is the selective reduction of a complex mixed phase oxide such as red mud and extraction of a single, almost pure target product. ‘We did a couple of thermodynamic calculations to show in which excitation energy spectrum we had to work to carve out the iron and not the other elements,’ he says. ‘Iron is the easiest to extract material.’ In future, Raabe says he would like to extend the technique to see whether the trace rare-earth elements present in some red muds can also be extracted.

Source: © Samuel Kubani/AFP/Getty Images
An industrial accident in 2010 flooded several Hungarian towns with red mud, leading to 10 deaths and costing millions to clean up
The process could be highly energy efficient, says Raabe, because the reaction between hydrogen radicals and oxides is exothermic. Therefore, once the plasma is ignited, the reaction supplies the energy to keep the oxides molten and the only energy input needed is the electricity to maintain the arc. Unlike coke, this can be produced renewably. If the hydrogen is also renewable, the process becomes truly zero carbon, but Raabe acknowledges that to produce all the steel required each year by this process would require 10,000 times more green hydrogen than is currently available globally. However, he points out that the use of a plasma process uses as little hydrogen as possible by maximising its reactivity. He also believes that the potential to renewably produce a valuable end product at all could help to make the detoxification of red mud economically viable.
Ray Peterson, Director of Technology at the US aluminium recycling company Real Alloy, is impressed by the science but cautions that the process is still a long way from industrial application. ‘They have shown the process is possible, they have a byproduct that appears to be inert, they’re getting iron metal out, so that’s all good,’ he says. ‘To make the process work they’re going to need cheap electricity and cheap hydrogen, which everybody wants right now, but is not available,’
‘The problem is you’re competing against conventional processes already out there for making iron,’ adds Peterson. ‘Granted, they all have a carbon footprint, but until we get a carbon tax or some other incentive to go down this route [the opportunities for using this process are ] probably going to be limited,’ he concludes.
References
M Jovičević-Klug et al, Nature, 2024, DOI: 10.1038/s41586-023-06901-z
No comments:
Post a Comment