US Administration sets out rules for clean hydrogen credits
10 January 2024
The White House says its tax credit incentive stands to be the most consequential policy supporting the deployment of clean hydrogen in US history - but stipulations that would make credits available only to the newest clean electricity generating units would exclude most of the US nuclear fleet.
.jpg?ext=.jpg) (Image: Pixabay)
(Image: Pixabay)The proposed regulations for claiming the so-called 45V Clean Hydrogen Production Tax Credit established under the 2022 Inflation Reduction Act issued by the US Treasury Department and Internal Revenue Service were published in the Federal Register on 26 December.
The Inflation Reduction Act (IRA) of 2022 provides a production credit for each kilogram of qualified clean hydrogen produced by a taxpayer at a qualified clean hydrogen production facility. Providing tax credits of up to USD3 per kilogram of hydrogen to projects with low lifecycle greenhouse gas emissions, the White House says the 45V credit accompanies other hydrogen programmes such as the Department of Energy's Regional Clean Hydrogen Hubs Program.
The credit amount is dependent on the emissions intensity of the hydrogen production process. However, the proposed regulations stipulate that only clean power generators that began operating within three years of the hydrogen facility entering service will be eligible.
This requirement effectively eliminates all existing US clean energy generating capacity from qualifying for the credit, instead requiring hydrogen producers to construct purpose-built energy projects for their production facilities, Nuclear Energy Institute (NEI) President Maria Korsnick said.
"This requirement will make many clean hydrogen projects uneconomic and will create years of delay for the few projects that can move forward in the face of the Administration's added constraints," she added. "The US nuclear fleet is well positioned to propel the US as a global leader in clean hydrogen production, but the Administration's proposal will undercut the development of a domestic clean hydrogen economy and will bolster the competitiveness of our global competitors.
"By explicitly allowing the zero-emission nuclear power production tax credit to be claimed in conjunction with the H2 Credit, the authors of the IRA made clear that existing nuclear facilities were eligible for the H2 Credit. NEI will continue to engage the Administration to ensure the H2 credit is implemented as clearly intended by Congress. Doing so will ensure nuclear energy can fill its full potential in decarbonising the hardest-to-abate sectors of our economy and will accelerate progress toward a cleaner energy economy."
Last October, US President Joe Biden and Energy Secretary Jennifer Granholm announced seven regional clean hydrogen hubs that will share USD7 billion in federal funding to accelerate the commercial-scale deployment of low-cost, clean hydrogen. Several hubs' plans envisage using nuclear energy, with Constellation Energy, a major participant in the MachH2 hub, planning to build the world's largest nuclear-powered clean hydrogen production facility at its LaSalle Clean Energy Center in Illinois.
That facility will cost an estimated USD900 million, produce an estimated 33,450 tonnes of clean hydrogen per year and create thousands of "good-paying" jobs, but will need the tax credits to be cost-effective.
"The proposed rule flies in the face of Congress's clear intent to use America's nuclear energy to produce hydrogen," the company told the American Nuclear Society.
Senator Joe Manchin, chairman of the US Senate Energy and Natural Resources Committee, said the proposed rule "makes absolutely no sense", imposing onerous rules not included in the IRA that limit the ability of the credit to help develop a domestic hydrogen market. The proposed rules "will only make it more difficult to jumpstart the hydrogen market, which will be a critical part of our secure energy future", he said.
The proposed regulations are open to public comment until 26 February, and a public hearing will be held on 25 March.
Researched and written by World Nuclear News
New Discovery Overcomes Major Hurdle in Hydrogen Energy Economy
- The new material, a lanthanum hydride compound modified with strontium and oxygen, allows high-rate conduction of hydride ions at room temperature.
- This development overcomes previous limitations requiring water and continuous hydration in hydrogen fuel cells, simplifying design and reducing costs.
- The breakthrough promises to enhance the safety, efficiency, and energy density of hydrogen-based energy solutions, marking a significant step towards a viable hydrogen energy economy.
- Join Our Community
Its a breakthrough that means the advantages of hydrogen-based solid-state batteries and fuel cells are within practical reach, including improved safety, efficiency, and energy density, which are essential for advancing towards a practical hydrogen-based energy economy.
The study paper was published in the scientific journal Advanced Energy Materials.
For hydrogen-based energy storage and fuel to become more widespread, it needs to be safe, very efficient, and as simple as possible. Current hydrogen-based fuel cells used in electric cars work by allowing hydrogen protons to pass from one end of the fuel cell to the other through a polymer membrane when generating energy.
Efficient, high-speed hydrogen movement in these fuel cells requires water, meaning that the membrane must be continually hydrated so that it does not dry out. This is a constraint that adds an additional layer of complexity and cost to battery and fuel cell design that limits the practicality of a next-generation hydrogen-based energy economy.
To overcome this problem, scientists have been struggling to find a way to conduct negative hydride ions through solid materials, particularly at room temperature.
Kobayashi has said, “We have achieved a true milestone. Our result is the first demonstration of a hydride ion-conducting solid electrolyte at room temperature.”
The team had been experimenting with lanthanum hydrides (LaH3-δ) for several reasons; the hydrogen can be released and captured relatively easily, hydride ion conduction is very high, they can work below 100° C, and have a crystal structure.
But, at room temperature, the number of hydrogen atoms attached to lanthanum fluctuates between 2 and 3, making it impossible to have efficient conduction. This problem is called hydrogen non-stoichiometry, and was the biggest obstacle overcome in the new study.
When the researchers replaced some of the lanthanum with strontium (Sr) and added just a pinch of oxygen – for a basic formula of La1-xSrxH3-x-2yOy, they got the results they were hoping for.
The team prepared crystalline samples of the material using a process called ball-milling, followed by annealing. Then they studied the samples at room temperature and found that they could conduct hydride ions at a high rate.
Then, they tested its performance in a solid-state fuel cell made from the new material and titanium, varying the amounts of strontium and oxygen in the formula. With an optimal value of at least 0.2 strontium, they observed complete 100% conversion of titanium to titanium hydride, or TiH2. This means that almost zero hydride ions were wasted.
Kobayashi noted, “In the short-term, our results provide material design guidelines for hydride ion-conducting solid electrolytes. In the long-term, we believe this is an inflection point in the development of batteries, fuel cells, and electrolytic cells that operate by using hydrogen.”
The next step will be to improve performance and create electrode materials that can reversibly absorb and release hydrogen. This would allow “storage batteries” to be recharged, as well as make it possible to place hydrogen in storage and easily release it when needed, which is a requirement for hydrogen-based energy use.
***
This is a remarkable breakthrough in making fuel cells more practical. Not quite noted above is that today’s designs need an above freezing environment. All the time, not just when running. For much of the time and much of the world that is killer. Moreover a freeze would destroy a fuel cell, thus even a rare freeze zone would entail considerable risk. The team hasn’t said anything specific yet. But this is promising.
With that in mind this team’s work looks like quite a breakthrough. Now if the storage issue could just get a big cost and safety breakthrough . . .
By Brian Westenhaus via New Energy and Fuel
Scientists create DNA hydrogel-based, solar-powered evaporation system for highly efficient seawater desalination
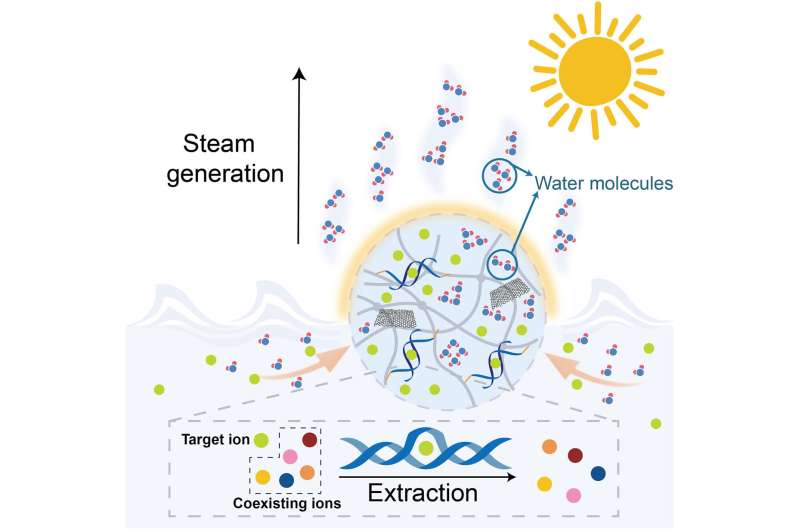
Minerals as well as freshwater can be obtained by desalinating seawater with solar power facilities for the sustainable development of human civilization. For instance, hydrogels have shown great power for solar-powered water evaporation potential, although the highly efficient and specific target extraction method remains to be expanded.
In a recent report published in Science Advances, Hanxue Liang and a team of researchers at the college of chemistry, and materials science in China, describe the process of highly efficient seawater desalination and the specific extraction of uranium with smart DNA hydrogels.
The DNA hydrogels promoted the evaporation of water, and the uranyl-specific DNA hydrogel exhibited a high capture capacity of 5.7 mg per gram for uranium from natural seawater due to rapid ion transport driven by solar-powered interfacial evaporation and high selectivity. These developments could enable easy-to-use devices suited for future seawater treatment.
Seawater desalination
Human society can be developed sustainably through access to sufficient freshwater and energy. The past few decades have witnessed the growing scarcity of freshwater as a threat to developing society, where rapid population and economic growth too have posed challenges to sustainable development.
To facilitate access to freshwater, life scientists have used ocean resources such as seawater desalination to account for up to 97% of the total water content on Earth.
Researchers have developed solar-powered seawater desalination as a promising method to produce seawater without additional energy consumption. Alongside desalination of seawater, a variety of valuable minerals and resources rich in the ocean can be extracted simultaneously, including uranium and lithium.
Hydrogels are soft materials made of hydrophobic 3D crosslinked polymer networks with high quantities of water, with a soft unique nature, versatility, and excellent biocompatibility.
Water evaporation
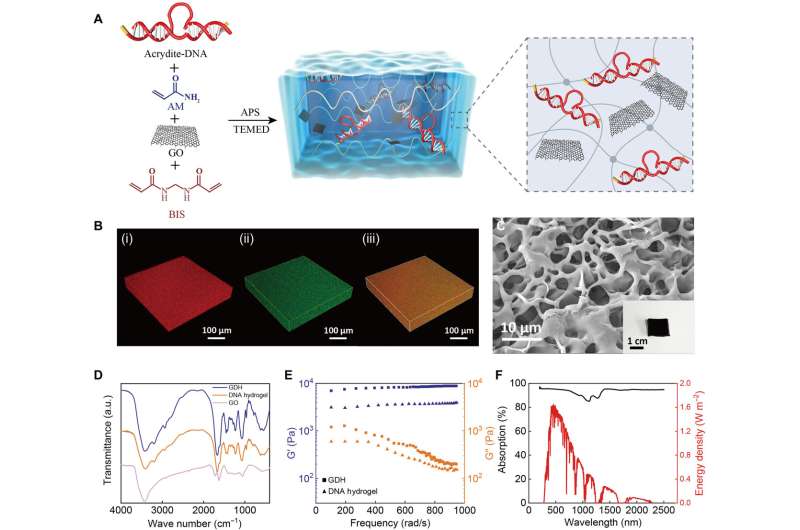
Hydrogel-based biomaterials have an ultrahigh water evaporation rate. In this work, Liang and team created a DNA hydrogel-based, solar-powered evaporation system to produce freshwater and target metal ion extraction from natural seawater. The scientists synthesized DNA hydrogels made of functional DNA-tethered polyacrylamide networks through a one-step copolymerization process.
The researchers introduced graphene oxide (GO) into the hydrogel to create GO-loaded DNA hydrogels that were easily recycled by a simple, thermally-driven elution method to test the feasibility of producing freshwater, and to extract valuable metal ions from natural seawater.
Characterizing the graphene-oxide-loaded DNA hydrogels (GDH)
Liang and colleagues prepared the graphene-oxide loaded DNA hydrogels in a one-step copolymerization reaction by using acrylamide, N,N'-methylenebisacrylamide, acrydite-modified DNA, with graphene-oxide added before polymerization.
The researchers applied confocal microscopy to investigate the microstructures of the as-prepared materials. The team noted the improved mechanical properties of the DNA hydrogel upon introducing graphene oxide. The addition of the compound into the DNA hydrogel enabled highly efficient solar light-absorbing properties. The materials showed broadband and a highly efficient absorption wavelength range.
The experiments
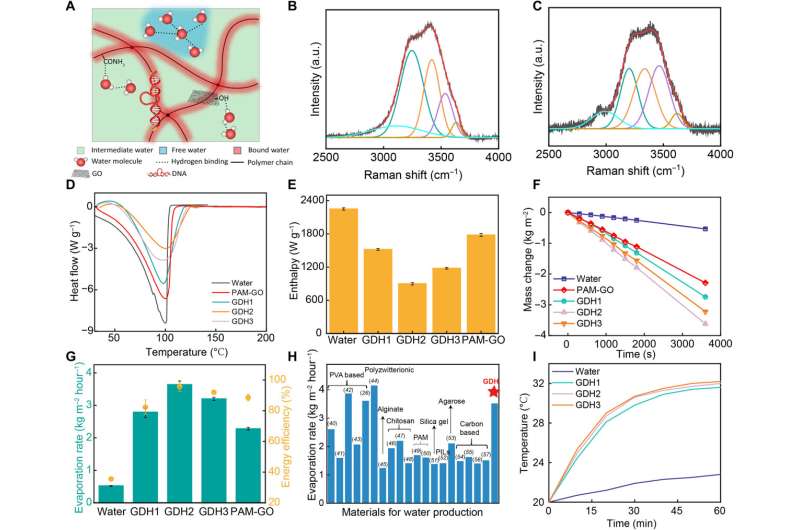
The research team explored the competence of the biomaterials for highly efficient solar-powered water evaporation. Where the materials formed hydrogen bonds with hydrophilic polymer chains to confine water molecules within a hydrophilic hydrogel construct.
The process led to the formation of three types of water molecules, including free water, intermediate water, and bound water, where the process required less energy for intermediate water to escape the hydrogel than for free water to transit from the bulk liquid phase.
Hydrophilic groups in the material facilitated the activation of water molecules and reduced the evaporation energy of water by regulating the hydrogen bonding structures of encapsulated water molecules. The researchers used Raman spectroscopy to differentiate varieties of water structures in pure water and within the hydrogel material.
Graphene-oxide loaded DNA hydrogels to extract uranium
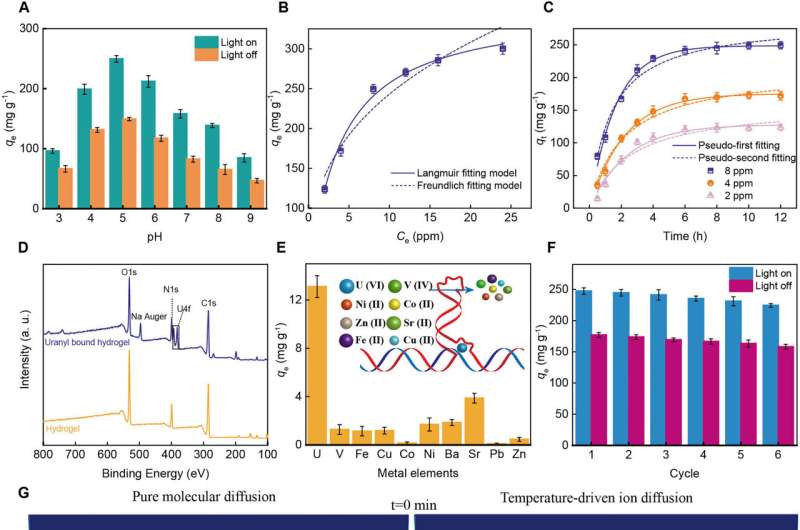
Uranium is distributed in oceans as a key element in nuclear fuels with magnitudes of abundance when compared to land. The main form of uranium in seawater is uranyl ions, with a low concentration, where the elements coexist with many interfering ions including vanadium.
DNAzymes or catalytic DNA that contains short DNA oligonucleotides can extract rare metal ions such as uranyl ions, due to their high affinity and specific metal ion binding properties.
To facilitate the process, the team encoded the DNA units that were incorporated into the graphene-oxide hydrogel with the sequence of uranyl-selective DNAzyme to extract the uranyl ions. The control experiments showed how the hydrogel without DNA had a much lower uranium adsorption capacity.
The biomaterial was also capable of selectivity for uranium in the presence of vanadium ions, and the incorporation of DNA provided a key for selectivity with uranium adsorption. The outcomes outlined the wide range of anti-biofouling activity of the biomaterial for long-term applications in seawater desalination and during uranium extraction.
Further experiments and extracting uranium from natural seawater
Liang and team performed numerical simulations to compare the migration of ions through diffusion, and noted the presence of a temperature gradient upon illumination. The temperature gradient increased the transport of ions to promote the process in the entire hydrogel construct.
The DNA units in the biomaterial selectively extracted diverse targets to establish a lithium ion-dependent DNAzyme, as well as sodium, potassium, and magnesium metal ion extraction with smart DNA hydrogels.
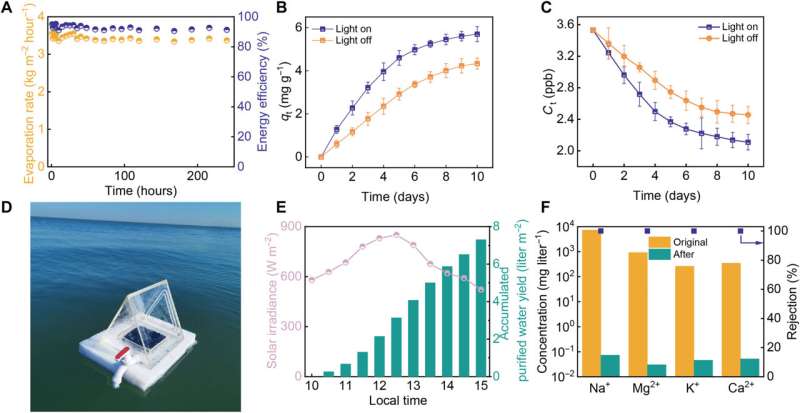
While the graphene-oxide loaded DNA hydrogel biomaterial showed excellent capacity for uranyl extraction from natural seawater, the extraction capacity increased under solar illumination in an endothermic process. The uranium extraction performance with the biomaterial was as competent as that obtained with advanced extraction materials.
Liang and team tested the performance of the biomaterial with natural seawater via inductively coupled plasma optical emission spectroscopy in the Bohai sea. The outcomes showed the capacity to simultaneously evaporate water ultrafast and highly selectively from natural seawater with the solar-powered biomaterial-integrated device.
Outlook
In this way, Hanxue Liang and team invented a DNA hydrogel-based, solar-powered evaporation system to simultaneously facilitate high-speed seawater desalination and highly specific extraction of minerals including uranium and lithium. The highly hydrophilic network structures facilitated water evaporation enthalpy in the hydrogel to effectively promote solar-powered seawater evaporation.
The team introduced DNA structures to improve the evaporation efficiency of the platform and facilitated the hydrogel with specific metal ion extraction properties for metal ion extraction under illumination. The smart DNA hydrogels are promising for freshwater harvesting and uranyl extraction from seawater, and for uranyl-enriched nuclear wastewater treatment.
More information: Hanxue Liang et al, Solar-powered simultaneous highly efficient seawater desalination and highly specific target extraction with smart DNA hydrogels, Science Advances (2023). DOI: 10.1126/sciadv.adj1677
Journal information: Science Advances
© 2024 Science X Network
No comments:
Post a Comment