USDA announces new milk testing order for H5N1 bird flu

The highly pathogenic avian influenza H5N1 was first found in dairy cattle in the United States in March 2024. File Photo by Ian Wagreich/UPI | License Photo
Dec. 6 (UPI) -- The U.S. Department of Agriculture Friday announced a new national milk testing order to check for H5N1 bird flu. It requires raw unpasteurized milk samples nationwide to be collected and shared with the USDA for testing.
Highly pathogenic avian influenza H5N1 was first found in dairy cattle in the United States in March 2024.
"Since the first HPAI detection in livestock, USDA has collaborated with our federal, state and industry partners to swiftly and diligently identify affected herds and respond accordingly," said Agriculture Secretary Tom Vilsak in a statement. "This new milk testing strategy will build on those steps to date and will provide a roadmap for states to protect the health of their dairy herds."
In April 2024, the USDA said the novel movement of H5N1 from wild birds to dairy cows required further testing.
Vilsack said the new federal order for milk testing, "will give farmers and farmworkers better confidence in the safety of their animals and ability to protect themselves, and it will put us on a path to quickly controlling and stopping the virus' spread nationwide."
The new federal order consists of three requirements.
Raw milk samples must be shared upon request from milk producing, dairy processing and transport entities.
Second, herd owners with positive cattle must provide epidemiological information that enables activities such as contact tracing and disease surveillance.
The final requirement is for private laboratories and state veterinarians to report positive results to USDA that come from tests done on raw milk samples drawn as part of the new milk testing order.
The new order is part of a multi-stage plan to work with states to stop the spread of H5N1 bird flu among U.S. dairy herds.
It includes nationwide testing of milk silos to monitor for bird flu, determining a state's H5N1 dairy cattle status and detecting and responding to rapidly respond to virus infections.
The fourth stage, after all dairy herds in a state are considered to be unaffected, is to continue regular sampling of farms' bulk tanks so the disease does not re-emerge.
And the final stage will be to demonstrate freedom from H5 for U.S. dairy cattle.
Health and Human Services Secretary Xavier Becerra said in a statement, "This testing strategy is a critical part of our ongoing efforts to protect the health and safety of individuals and communities nationwide."
He added, "Our primary responsibility at HHS is to protect public health and the safety of the food supply, and we continue to work closely with USDA and all stakeholders on continued testing for H5N1 in retail milk and dairy samples from across the country to ensure the safety of the commercial pasteurized milk supply."
The first six states in the milk testing program will be California, Colorado, Michigan, Mississippi, Oregon, and Pennsylvania.
Washington became the sixth state to identify a human infection of avian flu in October. Outbreaks have ben found in poultry, dairy, cattle and wildlife.
At least 31 human cases among workers in contact with animals have occurred in Washington, California, Texas, Michigan, Colorado and Missouri.

The highly pathogenic avian influenza H5N1 was first found in dairy cattle in the United States in March 2024. File Photo by Ian Wagreich/UPI | License Photo
Dec. 6 (UPI) -- The U.S. Department of Agriculture Friday announced a new national milk testing order to check for H5N1 bird flu. It requires raw unpasteurized milk samples nationwide to be collected and shared with the USDA for testing.
Highly pathogenic avian influenza H5N1 was first found in dairy cattle in the United States in March 2024.
"Since the first HPAI detection in livestock, USDA has collaborated with our federal, state and industry partners to swiftly and diligently identify affected herds and respond accordingly," said Agriculture Secretary Tom Vilsak in a statement. "This new milk testing strategy will build on those steps to date and will provide a roadmap for states to protect the health of their dairy herds."
In April 2024, the USDA said the novel movement of H5N1 from wild birds to dairy cows required further testing.
Vilsack said the new federal order for milk testing, "will give farmers and farmworkers better confidence in the safety of their animals and ability to protect themselves, and it will put us on a path to quickly controlling and stopping the virus' spread nationwide."
The new federal order consists of three requirements.
Raw milk samples must be shared upon request from milk producing, dairy processing and transport entities.
Second, herd owners with positive cattle must provide epidemiological information that enables activities such as contact tracing and disease surveillance.
The final requirement is for private laboratories and state veterinarians to report positive results to USDA that come from tests done on raw milk samples drawn as part of the new milk testing order.
The new order is part of a multi-stage plan to work with states to stop the spread of H5N1 bird flu among U.S. dairy herds.
It includes nationwide testing of milk silos to monitor for bird flu, determining a state's H5N1 dairy cattle status and detecting and responding to rapidly respond to virus infections.
The fourth stage, after all dairy herds in a state are considered to be unaffected, is to continue regular sampling of farms' bulk tanks so the disease does not re-emerge.
And the final stage will be to demonstrate freedom from H5 for U.S. dairy cattle.
Health and Human Services Secretary Xavier Becerra said in a statement, "This testing strategy is a critical part of our ongoing efforts to protect the health and safety of individuals and communities nationwide."
He added, "Our primary responsibility at HHS is to protect public health and the safety of the food supply, and we continue to work closely with USDA and all stakeholders on continued testing for H5N1 in retail milk and dairy samples from across the country to ensure the safety of the commercial pasteurized milk supply."
The first six states in the milk testing program will be California, Colorado, Michigan, Mississippi, Oregon, and Pennsylvania.
Washington became the sixth state to identify a human infection of avian flu in October. Outbreaks have ben found in poultry, dairy, cattle and wildlife.
At least 31 human cases among workers in contact with animals have occurred in Washington, California, Texas, Michigan, Colorado and Missouri.
A new study finds tweaking part of the H5N1 virus infecting dairy cows in a single spot could allow it to better attach to human cell receptors, raising concerns it could transmit more easily between people
By Lauren J. Young
SCIENTIFIC AMERISAN
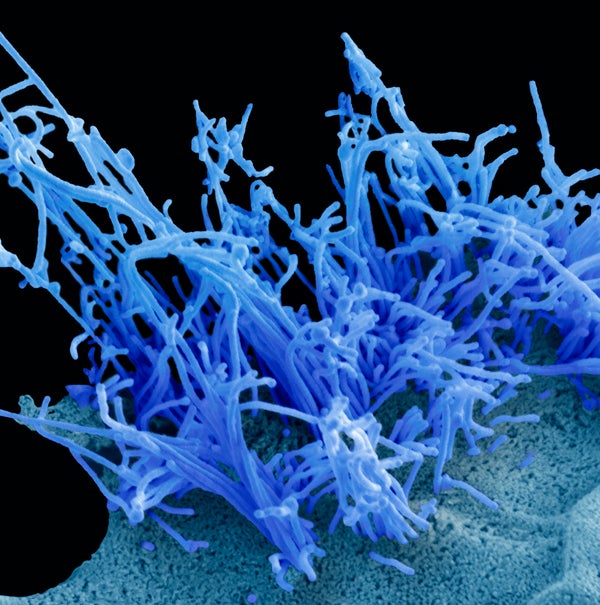
A human cell infected with the avian influenza virus H5N1 (blue filaments).
Steve Gschmeissner/Science Source Epidemiology
Scientists have discovered that H5N1, the strain of highly pathogenic avian influenza virus currently spreading in U.S. dairy cows, only needs a single mutation to readily latch on to human cells found in the upper airway. The findings, published today in Science, illustrate a potential one-step path for the virus to become more effective at human transmission—and could have major implications for a new pandemic if such a mutation were to become widespread in nature.
Avian influenza viruses are dotted with surface proteins that allow them to bind to bird cell receptors, which permit the virus to enter the cells. The cell receptors in birds are different from those in humans, but that variation is “very subtle,” says James Paulson, a study co-author and a biochemist at Scripps Research. “For a new pandemic H5N1 virus, we know that it has to switch receptor specificity from avian-type to human-type. So what will it take?” To his and his co-authors’ surprise, that switch only needed one genetic alteration.
The particular group, or clade, of H5N1 responsible for the current outbreak was first detected in North America in 2021 and has affected a wide range of animal populations, including wild birds, bears, foxes, a variety of marine mammals and, most recently, dairy cows. Since outbreaks of H5N1 in U.S. dairy herds began this spring, human cases have been mostly linked to sick poultry or cows, and the majority of human infections have been mild ones among farmworkers at high risk of exposure (with some notable exceptions). There haven’t been any signs of transmission between people—and the virus’s receptor binding preference is a key barrier to that.
“It’s obviously speculative, but the better the virus becomes at likely binding to human receptors—it’s not great because it’s going to probably lead to human-to-human transmission,” says Jenna Guthmiller, an immunologist at the University of Colorado Anschutz Medical Campus, who was not involved in the new research.
The study authors focused on altering one of H5N1’s surface proteins, hemagglutinin, which contains the binding site that allows the virus to latch onto host cell receptors and kick-start infection. The researchers generated viral proteins from genetic sequences of the virus isolated from the first human case in Texas, which occurred in a person who developed bird flu after exposure to an infected cow. No live virus was used in the experiment. Then the scientists engineered an assortment of different mutations into hemagglutinin’s chain of amino acids, or protein building blocks. A single mutation that swapped the 226th amino acid in the sequence for another allowed H5N1 to switch its binding affinity from receptors on bird cells to receptors on human cells in the upper respiratory tract.
Curated by Our Editors

Can Antarctic Wildlife Survive Another Deadly Bird Flu Season?
Meghan Bartels

Anthony Fauci Warns of Bird Flu Dangers—And How Public Division Could Make It Worse
Rachel Feltman, Tanya Lewis, Fonda Mwangi & Jeffery DelViscio
Bird Flu Has Infected Two Young People. Here’s Why Experts Are Concerned
Lauren J. Young
A Bird Flu Vaccine Might Come Too Late to Save Us from H5N1
Maggie Fox
Past research has shown that several influenza mutations, including the ones tested in the new paper, are important in human receptor binding, Guthmiller says. These genetic tweaks have been flagged in previous influenza virus subtypes that have caused human pandemics, such as those in 1918 and 2009. But past viruses typically required at least two mutations to successfully change their preference to human receptors, explains co-author Ian Wilson, a structural and computational biologist at Scripps. “This was surprising. It was just this single mutation [that] was sufficient to switch the receptor specificity,” he says.
Paulson adds that the particular mutation the scientists tested in the new study had previously been investigated during H5N1 outbreaks in poultry and some humans in 2010, but it didn’t affect the virus’s human receptor binding. “But the virus has subtly changed,” Paulson says. “Now that mutation does cause the change.”
Wilson and Paulson note the mutated H5N1 protein in their study bound weakly to human receptors but more strongly than the 2009 H1N1 virus, which caused the “swine flu” human pandemic. “The initial infection is what we’re concerned about to initiate a pandemic, and we believe that the weak binding that we see with this single mutation is at least equivalent to a known human pandemic virus,” Paulson says. The study did identify a second mutation in another area of hemagglutinin, the amino acid at position 224, that could further enhance the virus’s binding ability in combination with the 226 mutation.
Guthmiller isn’t surprised about the findings, given the 226 mutation’s known significance in flu receptor preference, but adds, “It’s never great when you see that it only really takes one mutation.” The study “also sort of provides us an idea of what we should be looking for and what sites of the hemagglutinin protein we should be focusing on to understand its potential to change and infect us better.”
A teenager in Canada was recently hospitalized in critical condition from bird flu with an unknown exposure. Genetic sequencing, which showed a strain of H5N1 that was similar to one circulating in Canadian poultry, detected mutations in two positions, one of which was at 226—the same position studied in the new paper. Scientists don’t know if either mutation was responsible for the teenager’s severe condition, but some expressed concern that the changes could be a sign of the virus potentially adapting to human cells.
Paulson says it’s too early to draw conclusions or parallels between the teenager’s case and the study findings. The amino acids the researchers tweaked in the study were not the same as those in the Canadian case’s viral sequence, for instance, he says. “There’s a lot of chatter that, ‘oh, my gosh, that amino acid is mutating,’ but there’s no evidence yet that that would actually give us the specificity that would be required for human transmission,” Paulson says. But he adds that the case is still significant.
Most bird flu cases in humans reported this year have been mild. In past outbreaks, H5N1 has caused severe respiratory disease because of its preference to bind to cells in the lower respiratory tract, Guthmiller explains. "You’re basically causing a viral pneumonia,” she says. “But if you increase binding to human receptors that are in the upper respiratory tract,” as this study did, “that’s more likely going to look more like your common cold–like symptoms.” That said, viruses that prefer the upper respiratory tract, including the nose and throat, are more likely to spread through coughing and sneezing, she says. That could lead to more spread through human contact.
Better receptor binding doesn’t necessarily cause disease on its own. Several other factors are important, such as the virus’s ability to replicate and proliferate in the body. But attaching to cells is an initial step, Paulson says. “The magic that we hope doesn’t happen is that all of those things come together so that we have that first [human-to-human] transmission and that becomes a pandemic virus,” he says.
Rights & Permissions
Lauren J. Young is an associate editor for health and medicine at Scientific American. She has edited and written stories that tackle a wide range of subjects, including the COVID pandemic, emerging diseases, evolutionary biology and health inequities. Young has nearly a decade of newsroom and science journalism experience. Before joining Scientific American in 2023, she was an associate editor at Popular Science and a digital producer at public radio’s Science Friday. She has appeared as a guest on radio shows, podcasts and stage events. Young has also spoken on panels for the Asian American Journalists Association, American Library Association, NOVA Science Studio and the New York Botanical Garden. Her work has appeared in Scholastic MATH, School Library Journal, IEEE Spectrum, Atlas Obscura and Smithsonian Magazine. Young studied biology at California Polytechnic State University, San Luis Obispo, before pursuing a master’s at New York University’s Science, Health & Environmental Reporting Program.
No comments:
Post a Comment