Deborah Cohen
BBC
 G
GIt is among the most delicate and controversial challenges in modern medicine - how to determine whether the benefits of puberty blockers (or drugs that delay puberty) outweigh the potential harms.
This question came to the fore in June 2023 when NHS England proposed that in the future, these drugs would only be prescribed to children questioning their gender as part of clinical research.
Since then, a new government has arrived in Westminster and Health Secretary Wes Streeting has said he is committed to "setting up a clinical trial" to establish the evidence on puberty blockers. The National Institute for Health and Care Research is expected to confirm soon that funding is in place for a trial.
The dilemma that remains is, how will such a trial work?
Eighteen months since the announcement there is still a lack of consensus around how the trial should be conducted. It will also need to be approved by a committee of experts who have to decide, among other things, whether what's being tested might cause undue physical or psychological harm.
But there is a second unanswered question that some, but by no means all, scientists have that is more pressing than the first: is it right to perform this particular trial on children and young people at all?
A rapid rise in referrals
When the Gender and Identity Development Service (GIDS) was established at London's Tavistock Clinic in 1989, it was the only NHS specialist gender clinic for children in England, and those referred there were typically offered psychological and social support.
Over the last 10 years, however, there has been a rapid increase in referrals - with the greatest increase being people registered female at birth. In a separate development, around the same time the approach of typically offering psychological and social support moved to one of onward referrals to services that prescribed hormone drugs, such as puberty blockers.
Known scientifically as gonadotropin-releasing hormone (GnRH) analogues, puberty blockers work on the brain to stop the rise in sex hormones - oestrogen and testosterone - that accompany puberty. For years, they were prescribed to young patients with gender dysphoria (those who feel their gender identity is different from their biological sex). But in March 2024, NHS England stopped the routine prescribing of puberty blockers to under 18s, as part of an overhaul of children's gender identity services.
NHS England said in a policy statement: "There is not enough evidence to support the safety or clinical effectiveness of PSH [puberty suppressing hormones] to make the treatment routinely available at this time."
The ban was later tightened to apply to private clinics as well.

PA
Dr Hilary Cass published her final report in April 2024
In April 2024, a review of gender identity services for children and young people, led by Dr Hilary Cass, a past president of the Royal College of Paediatrics and Child Health, published its final report, which called out the "field of gender care" for not taking a cautious and careful approach.
She also reported that the change in practice at GIDS away from one primarily relying on psychological and social support was largely based on a single study that looked at the effect of medical interventions such as puberty blockers on a very narrowly defined group of children and there was a lack of follow up in the longer term.
Elsewhere, some other countries were re-examining puberty blockers too. Scotland paused the use of them while Finland, Sweden, France, Norway, and Denmark have all re-evaluated their positions on medical intervention for under 18s - including puberty blockers - to differing degrees. In other places there is still support for the use of puberty blockers.
In medicine, when there is genuine uncertainty as to whether the benefits of a treatment outweigh the harms - called equipoise - some ethicists argue there's a moral obligation to scientifically study such treatments. But there are some from across the debate who don't think there is equipoise in this case.
The ethical dilemma at the heart of the trial
The BBC has learned details about the arguments going on around the concept of a trial and how it could look. Some argue that there is already evidence that puberty blockers can help with mental health, and that in light of this it would be unethical to perform a trial at all because this would mean some young people experiencing gender distress would not be given them.
The World Professional Association of Transgender Health (WPATH) has expressed their concern about the trial for this reason. They support the use of puberty blockers, cross-sex hormones and surgery. WPATH, who have faced increasing criticism of their guidelines from some clinicians, say that it is ethically problematic to make participation in a trial the only way to access a type of care that is "evidence based, widely recognised as medically necessary, and often reported as lifesaving."
Meanwhile other clinicians believe there is no good evidence that puberty blockers can help with mental health at all. They also point to research that questions the negative impact that the drugs might have on brain development among teenagers, as well as evidence around the negative impact on bone density.
Dr Louise Irvine is a GP and co-chair of the Clinical Advisory Network on Sex and Gender which says it is cautious about using medical pathways in gender dysphoric children. She says: "Given that puberty blockers by definition disrupt a crucial natural phase of human development, the anticipated benefits must be tangible and significant to justify the risk to children.
"In pushing ahead with a puberty blockers trial, we are concerned that political interests are being prioritised over clinical, ethical and scientific concerns, and over the health and wellbeing of children."
The NHS adult gender services holds data that tracks 9,000 young people from the youth service. Some argue that this should be scrutinised before any trial goes ahead as it could provide evidence on, among other things, the potential risks of taking puberty blockers.
But there is a third view held by some others, including Gordon Guyatt, a professor at McMaster University in Canada, who points out that randomised trials are done in "life-threatening stuff all the time" where no-one can be sure of the long-term effects of a treatment. In his view it would be "unethical not to do it".
"With only low quality evidence, people's philosophies, their attitudes or their politics, will continue to dominate the discussion," he argues. "If we do not generate better evidence, the destructive, polarised debate will continue."
Dr Hilary Cass published her final report in April 2024
In April 2024, a review of gender identity services for children and young people, led by Dr Hilary Cass, a past president of the Royal College of Paediatrics and Child Health, published its final report, which called out the "field of gender care" for not taking a cautious and careful approach.
She also reported that the change in practice at GIDS away from one primarily relying on psychological and social support was largely based on a single study that looked at the effect of medical interventions such as puberty blockers on a very narrowly defined group of children and there was a lack of follow up in the longer term.
Elsewhere, some other countries were re-examining puberty blockers too. Scotland paused the use of them while Finland, Sweden, France, Norway, and Denmark have all re-evaluated their positions on medical intervention for under 18s - including puberty blockers - to differing degrees. In other places there is still support for the use of puberty blockers.
In medicine, when there is genuine uncertainty as to whether the benefits of a treatment outweigh the harms - called equipoise - some ethicists argue there's a moral obligation to scientifically study such treatments. But there are some from across the debate who don't think there is equipoise in this case.
The ethical dilemma at the heart of the trial
The BBC has learned details about the arguments going on around the concept of a trial and how it could look. Some argue that there is already evidence that puberty blockers can help with mental health, and that in light of this it would be unethical to perform a trial at all because this would mean some young people experiencing gender distress would not be given them.
The World Professional Association of Transgender Health (WPATH) has expressed their concern about the trial for this reason. They support the use of puberty blockers, cross-sex hormones and surgery. WPATH, who have faced increasing criticism of their guidelines from some clinicians, say that it is ethically problematic to make participation in a trial the only way to access a type of care that is "evidence based, widely recognised as medically necessary, and often reported as lifesaving."
Meanwhile other clinicians believe there is no good evidence that puberty blockers can help with mental health at all. They also point to research that questions the negative impact that the drugs might have on brain development among teenagers, as well as evidence around the negative impact on bone density.
Dr Louise Irvine is a GP and co-chair of the Clinical Advisory Network on Sex and Gender which says it is cautious about using medical pathways in gender dysphoric children. She says: "Given that puberty blockers by definition disrupt a crucial natural phase of human development, the anticipated benefits must be tangible and significant to justify the risk to children.
"In pushing ahead with a puberty blockers trial, we are concerned that political interests are being prioritised over clinical, ethical and scientific concerns, and over the health and wellbeing of children."
The NHS adult gender services holds data that tracks 9,000 young people from the youth service. Some argue that this should be scrutinised before any trial goes ahead as it could provide evidence on, among other things, the potential risks of taking puberty blockers.
But there is a third view held by some others, including Gordon Guyatt, a professor at McMaster University in Canada, who points out that randomised trials are done in "life-threatening stuff all the time" where no-one can be sure of the long-term effects of a treatment. In his view it would be "unethical not to do it".
"With only low quality evidence, people's philosophies, their attitudes or their politics, will continue to dominate the discussion," he argues. "If we do not generate better evidence, the destructive, polarised debate will continue."
- Dr Cass found the existing research in the field was poor quality and that there was not a reliable enough evidence base to base clinical decisions on. Young people involved in many of the existing studies may have also had interventions including psychological support and other medical treatments and so it was not always possible to disentangle the effect of each different treatment.
- When it comes to suppressing puberty by using drugs, the rationale for doing so "remains unclear", Dr Cass said. One of the original reasons given was to allow time to think by delaying the onset of puberty. But the evidence suggests the vast majority who start on puberty blockers go on to take cross-sex hormones - oestrogen or testosterone. It is not clear why but one theory, the Cass report suggests, is that puberty blockers may, in their own right, change the "trajectory" of gender identity development.
- - Clinicians "are unable to determine with any certainty" which young people "will go on to have an enduring trans identity", Dr Cass wrote. In other words, there's a lack of clarity about which young people might benefit in the long term and which may be harmed overall by the process.
How the trial could look
Recruitment for the trial is due to start in 2025, months later than originally anticipated. Young people will likely be referred after a full assessment by specialist clinicians. A lot is still to be determined, including how many participants there will be.
Ultimately the scientists running the trials will need to establish whether people who get an intervention are better off than those who do not. In this case, do the puberty blocking drugs and their effect make the young people better off?
"Better off" in this instance includes the extent to which a young person's mental health may be improved if they are happy with their body. Quality of life is determined by various factors including self-confidence and self-esteem. As well as getting the personal views from the young people and parents, the trial could measure actual real life changes, such as time spent in education and time spent with family and friends.
But there are potential harms to study too, such as the possibility of reduced bone density. Some scientists suggest examining the impact on learning using a form of IQ test.
Normal brain development is influenced by both puberty and chronological age, which usually act in tandem during adolescence. It's not clear how this is affected when puberty is suppressed. Brain scans are one way of understanding any effect.
Some scientists believe it may be possible to simply randomly assign trial participants into two groups where one gets puberty blockers, the other gets a placebo and nobody is aware which group they're in.
But others believe a placebo group is impossible. They say the placebo group would go through puberty, realise they weren't on puberty blockers and potentially drop out of the trial or even find other ways to obtain puberty blockers. Either scenario would reduce the validity of the results.
Professor Gordon Guyatt and others have outlined a potential trial where the group of patients not receiving drugs would be made up entirely of children who are keen to socially transition, such as by changing how they dress and altering their name and pronouns. Researchers could then monitor the difference between the groups.
A second possibility is that both trial groups are given puberty blockers but one group gets them after a delay, during which time they receive psychological and emotional support. This would help researchers determine, among other things, whether their gender-related distress subsides during that delay while receiving the support.
Alongside this there would be a "matched" control group that doesn't take a placebo or puberty blockers, whether for health reasons or because they don't want to, that get similar tests and scans.
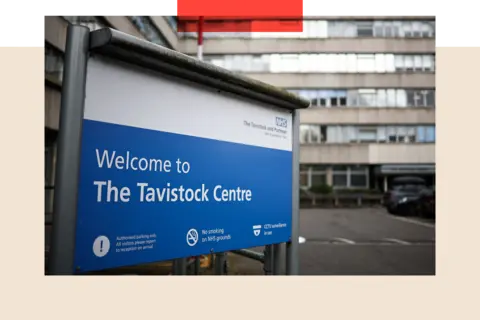
The Gender and Identity Development Service (GIDS) was established at London’s Tavistock Clinic in 1989
Puberty occurs in stages when different bodily changes occur. A third proposal could involve a second group being given drugs at a later stage in puberty than the first.
This would allow researchers to explore when the right time to give puberty blockers might be. For example, it would enable the researchers to see if starting the drugs early improves wellbeing by reducing gender-specific body changes. They would also be able to see whether starting the drugs earlier has a greater negative impact on bone density and brain development.
Children referred to GIDS also experienced higher rates of anxiety, depression, eating disorders, and autism compared to the general child population. Trial participants would continue to receive treatment related to these conditions but - so we know any differences in the results from the groups are down to the drug - they will need to be balanced for the above conditions.
All these considerations demonstrate the complexity of trying to obtain evidence in this area that is reliable and definitive.
What parents say
Many parents are watching closely to see how it will play out. Annabel (not her real name) is one of them. She is part of the Bayswater Group, a collection of parents with children who are questioning their gender who say they are "wary of medical solutions to gender dysphoria". She began looking into puberty blockers when her own daughter began questioning her gender in her early teens, an option put on the table by GIDS.
Ultimately her daughter decided not to take them. Annabel was not convinced there was enough evidence to show they were beneficial and she was unsure what it would mean for her daughter's long-term physical and psychological health.
Today, she still has unanswered questions - including some further ones around the trial. "A big concern for me is will this new trial, if it gets approval, give us the evidence that we want? Or will we end up with more weak data that Dr Cass said undermined decision making in this area?"
Natacha Kennedy, a lecturer at Goldsmiths, University of London who researches transgender issues, has examined the results of a survey of 97 parents of young people with gender-related distress that took place following the puberty blockers ban. She believes that puberty blockers should be an option available for young people questioning their gender and that many will not accept being part of a placebo group in a trial.
"These parents are desperate and if [they] get to a trial and it turns out their child is not being given the actual puberty blockers, then there is no point in them being there," she says.
"There may be some parents who would… find another way [to obtain the drugs]."
Whatever trial format is settled on, more scrutiny will follow. And there will no doubt be fierce debate about the merits of the trial and what it can tell us, as many scientists around the world are watching to see what happens in the UK.
But inevitably, there will be a long wait to fully understand the longer term effects on physical and mental health of those who take puberty blockers - and the long-term effects on those with gender-related distress who don't. Nor do we know how many people detransition, though the Cass report says, "there is suggestion that numbers are increasing".
"We really need to have long-term follow up," argues Annabel. "Can a child possibly understand what that means to their fertility and a loss of sexual function and what that will mean for their future life?"
For now, she and the scores of parents, carers and young people, can only watch and wait for the trial to begin and for its verdict - and what that means for whether puberty blockers will be prescribed to children once again in the future.

Deborah Cohen is a former BBC Newsnight health correspondent and is a Visiting Senior Fellow at LSE Health.
Top image: Getty

No comments:
Post a Comment