Vaccine Nationalism Is Doomed to Fail
Countries seeking to inoculate their citizens at the expense of everyone else are chasing a false promise.
/media/img/mt/2020/12/Atlantic_vac_nat_v1/original.png)
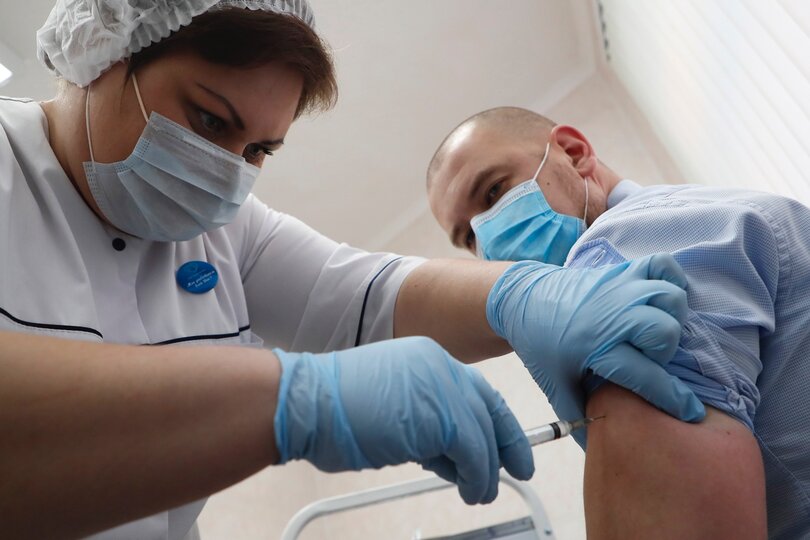
After nearly a year of waging a “war” on the coronavirus, many countries are poised to declare victory. Britain, which today became the first country to roll out the Pfizer and BioNTech vaccine, dubbed the start of its immunization program “V-Day,” echoing the language used to commemorate its win in World War II. Elsewhere, China and Russia have already begun distributing their own state-backed vaccines for domestic use. And although the United States has yet to grant regulatory approval to any of the vaccine candidates, Donald Trump was quick to claim the spate of positive vaccine news as his own.
Many of these vaccines, and the ongoing trials for potential alternatives, have benefited from huge levels of government investment—much of it coming from wealthy countries determined to secure their spot at the front of the line for when the vaccines are finally ready. To that end, billions of doses were reserved before any had been approved for use, with many countries claiming enough to inoculate their population several times over.
This “vaccine nationalism,” in which countries prioritize their domestic needs at the expense of others, may have helped accelerate efforts to develop such drugs, but it is already showing its limits. With wealthy countries claiming the lion’s share of prospective doses for themselves, and with global efforts to equalize vaccine distribution facing enduring unilateralism and limited resources, a coronavirus vaccine returning the world to something resembling “normal” could take considerable time—perhaps even longer than it needs to. (Many of the governments warning against the unilateral approach are striking their own bilateral deals with vaccine manufacturers, in an apparent effort to have it both ways.) Without equal vaccine distribution, public-health experts warn, the pandemic could continue to live on residually for years, bringing with it even more death and further economic collapse. If the virus remains endemic anywhere, it will continue to pose a threat everywhere.
To better understand the extent to which richer countries have dominated the prospective-vaccine supply, it helps to look at the numbers. According to the Duke Global Health Innovation Center, which is tracking vaccine procurement worldwide, high- and upper-middle-income countries have collectively reserved nearly 5 billion vaccine doses. These doses are largely the product of bilateral deals between governments and vaccine makers, known as “advance market commitments,” in which governments commit to purchasing doses up front in exchange for priority access once the vaccine is approved. The U.S., for example, has entered into at least six of these bilateral deals, totaling more than 1 billion doses—more than enough to inoculate the entire American population. The European Union, Britain, and Canada have each entered into seven bilateral deals, with the potential of securing enough doses to cover their populations two, four, and six times over, respectively, the Duke numbers show.
In doing so, their governments are ensuring that even if one or more of the vaccine trials fails, they’ll still have plenty of other vaccines to fall back on. Put another way, they are “basically buying themselves a ticket to the front of the queue,” Clare Wenham, an assistant professor of global-health policy at the London School of Economics, told me. (Though wealthy countries such as Spain and Canada have suggested that they would be willing to donate excess doses to nations that need them, that process isn’t so simple. Before rolling out a vaccine, countries need to be able to ensure that they can foot the bill in the event of unexpected side effects or other costly issues—a liability that many poorer nations might not be able to afford.)
Countries seeking to acquire enough doses to inoculate their population isn’t in itself the problem. Governments, after all, have a responsibility to ensure the safety of their citizens. Nor have these bilateral agreements been entirely negative. “It has infused an absolutely astounding amount of money and investment into the development and manufacturing of these vaccines,” Andrea Taylor, the assistant director of programs at Duke’s Global Health Innovation Center, told me.
Ugur Sahin, the co-founder and CEO of BioNTech, told the Financial Times that Pfizer and BioNTech would give precedence to countries where its vaccine has received regulatory approval—which includes Britain. Moderna, meanwhile, is expected to prioritize the U.S. market. AstraZeneca has said that its first doses will be earmarked for Britain, owing to its partnership with Oxford University. The French pharmaceutical giant Sanofi, which is still in the early stages of its vaccine development, pledged that it would give the U.S. priority access in honor of the country’s early investment (a promise that was later walked back after causing ire in France).
“The number of doses we can actually get out in the world is very limited,” Taylor said. “When high-income countries take a large slice of that pie, there’s less pie for everyone else.”
In an ideal world, the pie would be shared equitably across nations, ensuring that no country is precluded from access to these lifesaving resources. It’s a goal that COVAX, an international alliance established to ensure that all countries have equal access to the vaccine, believes it can achieve if countries are willing to work together. But cooperation of this kind hasn’t exactly been a hallmark of public-health crises, past or present: During the 2009 swine-flu outbreak, wealthy countries reserved huge numbers of vaccine doses for themselves, leaving low-income countries to rely on donations that would arrive much later. Today, countries are still reverting to those nationalist instincts by imposing export bans on face masks, ventilators, and other essential resources.
COVAX hasn’t been without success: More than 180 economies, about half of which are high-income, have signed on to the initiative. What’s more, it has reserved enough manufacturing capacity to produce more than 1 billion doses—a goal which it aims to double by the end of next year. But that production will take time, leaving the alliance with just 700 million doses in advance market commitments in the short term—a sum significantly smaller than the number of doses reserved by many wealthy nations, and far short of its goal of providing enough doses to inoculate at least 20 percent of participating countries’ populations.
This isn’t COVAX’s only challenge. For one, the U.S. and Russia have both opted out of the project—a decision that, in the case of Washington, was prompted by the participation of the World Health Organization, which the U.S. withdrew from this year. (Though President-elect Joe Biden has pledged that the U.S. will rejoin the institution under his administration, it’s not yet clear whether that intent will extend to joining COVAX.) Then there’s the paradox that participating in COVAX hasn’t precluded high-income countries from striking their own bilateral vaccine deals—efforts that fundamentally undermine the initiative’s goal of equal distribution.
“There seems to be no stipulation that they shouldn't be doing these bilateral deals, so there is a tension there in [terms of] sheer goals,” Tamaryn Nelson, an Amnesty International researcher on the right to health, told me. “Clearly one undermines the other.”
“A collective response … doesn’t just make moral sense—it makes scientific sense,” a spokesperson for the vaccine alliance Gavi, which leads COVAX with the WHO, told me in an email. “If rich countries monopolize vaccines at the outset, it will take us a lot longer, and many more people will die, than if we distribute on a global, equitable basis.”
If the pandemic has cast doubt on the ability of countries to implement a multilateral response to a global problem, that portends poorly for other shared crises—not least climate change. Both challenges require unprecedented collaboration. Both disproportionately affect low-income communities. Neither can be averted by any single nation on the basis of wealth alone.
YASMEEN SERHAN is a London-based staff writer at The Atlantic.