Russian vaccine launch shocks scientists
Sputnik V to be approved without large human trial data
BY ANTHONY KING 14 AUGUST 2020 CHEMISTRYWORLD.COM
Russia’s unexpected authorisation of a largely untested Covid-19 vaccine has shocked researchers. Full scale production of the vaccine is to begin in September, with initial doses going to healthcare workers and vulnerable populations, followed by national rollout in January 2021.
The vaccine, developed at the Gamaleya Research Institute in Moscow, has apparently been tested on just 76 people. No data from animal or clinical studies have been published on potential efficacy or safety.
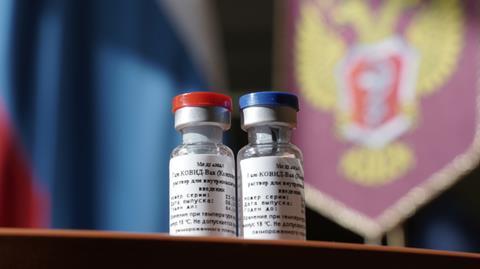
Russia coronavirus vaccine vials
Source: © Russian Health Ministry/Handout/Anadolu Agency/Getty Images
Rolling out the Sputnik V vaccine without the normal trials is a spectacular gamble when it comes to both its effectiveness at preventing the disease, and its potential for side effects
This is in stark contrast to even the most accelerated clinical programmes for other vaccines, in which phase 3 trials will recruit tens of thousands of volunteers to try and establish safety and effectiveness. Such large trials are also needed to spot uncommon side effects, which can quickly become a serious issue when a vaccine is given to millions of people. ‘Vaccine trials are harder to do in terms of safety and effectiveness because you must wait for people to get exposed,’ says epidemiologist Daniel Salmon, director of the Institute for Vaccine Safety at Johns Hopkins University, US. ‘It is really in phase 3 trials where you get a much better understanding of safety.’
Vaccines shouldn’t be the domain of Cold War political dispute
President Vladimir Putin’s televised announcement of the approval, dubbed Sputnik V, has an air of political point-scoring to it. ‘Putin says he will give it to other countries in October or November, which is just as we have our elections [in the US],’ says Hildegund Ertl, vaccine researcher at the Wistar Institute in Philadelphia,US, who is suspicious of this timing. ‘[Donald Trump] keeps saying we will have a vaccine by November.’ She hopes that the US and other countries will not tolerate political pressure on scientists to rush approval of vaccines. ‘Vaccines shouldn’t be the domain of Cold War political dispute,’ agrees Danny Altmann, immunologist at Imperial College London, UK. ‘This is incredibly unhealthy. The [potential] downsides are spectacular at every level.’
The Gamaleya vaccine trial used human adenoviruses to deliver RNA encoding the viral spike protein. The official website for Sputnik V describes its adenoviral vector technology as having been in development since the 1980s, with numerous studies showing effectiveness and safety. However, while Russia licensed vaccines using adenoviral vectors for Middle East Respiratory Syndrome (Mers) and Ebola, there were no large phase 3 studies for them. ‘Neither the adenovirus vectors nor the RNA vaccine have been licensed for any disease [in Western countries],’ notes Ertl. ‘Some have undergone large scale clinical trials, but we have zero experience in mass vaccinations.’
The Russian vaccine uses two different viral vectors, human adenovirus type 26 (Ad26) in an initial dose, and human adenovirus type 5 (Ad5) in a booster three weeks later. Both normally cause common colds, so a proportion of people will already have antibodies to them. That may lessen the vaccine’s effectiveness at stimulating an immune response against the Sars-CoV-2 virus. Ad26 is the same vector being employed by Johnson & Johnson (J&J), while China’s CanSino Biologics is using Ad5.
In principle, Ertl in principle likes the Russian approach of using two vectors. ‘It has a chance of being efficacious, and a reasonable chance of being safe. I just wish they would do a phase 3 trial like everyone else,’ she says. ‘It would take them another two or three months and then I would say great, wonderful job. But this way, I am just shaking my head, like every other scientist in this country.’
If the Russian gamble fails, it could damage public acceptance of other Covid-19 vaccines. ‘If there is a surprise, an adverse reaction which shows up once you inject 20,000 people, then people who wanted the vaccine will change their minds and we will never get a handle on this pandemic, until we have herd immunity in about 20 years,’ says Ertl. Such a failure could also impact acceptance of the vaccines that share the same vectors.
Salmon says he is not worried about European or US regulators pushing the limits in terms of vaccine safety, but has concerns about politicians who ‘don’t always appreciate the value of science’.
Russia’s unexpected authorisation of a largely untested Covid-19 vaccine has shocked researchers. Full scale production of the vaccine is to begin in September, with initial doses going to healthcare workers and vulnerable populations, followed by national rollout in January 2021.
The vaccine, developed at the Gamaleya Research Institute in Moscow, has apparently been tested on just 76 people. No data from animal or clinical studies have been published on potential efficacy or safety.

Russia coronavirus vaccine vials
Source: © Russian Health Ministry/Handout/Anadolu Agency/Getty Images
Rolling out the Sputnik V vaccine without the normal trials is a spectacular gamble when it comes to both its effectiveness at preventing the disease, and its potential for side effects
This is in stark contrast to even the most accelerated clinical programmes for other vaccines, in which phase 3 trials will recruit tens of thousands of volunteers to try and establish safety and effectiveness. Such large trials are also needed to spot uncommon side effects, which can quickly become a serious issue when a vaccine is given to millions of people. ‘Vaccine trials are harder to do in terms of safety and effectiveness because you must wait for people to get exposed,’ says epidemiologist Daniel Salmon, director of the Institute for Vaccine Safety at Johns Hopkins University, US. ‘It is really in phase 3 trials where you get a much better understanding of safety.’
Vaccines shouldn’t be the domain of Cold War political dispute
President Vladimir Putin’s televised announcement of the approval, dubbed Sputnik V, has an air of political point-scoring to it. ‘Putin says he will give it to other countries in October or November, which is just as we have our elections [in the US],’ says Hildegund Ertl, vaccine researcher at the Wistar Institute in Philadelphia,US, who is suspicious of this timing. ‘[Donald Trump] keeps saying we will have a vaccine by November.’ She hopes that the US and other countries will not tolerate political pressure on scientists to rush approval of vaccines. ‘Vaccines shouldn’t be the domain of Cold War political dispute,’ agrees Danny Altmann, immunologist at Imperial College London, UK. ‘This is incredibly unhealthy. The [potential] downsides are spectacular at every level.’
The Gamaleya vaccine trial used human adenoviruses to deliver RNA encoding the viral spike protein. The official website for Sputnik V describes its adenoviral vector technology as having been in development since the 1980s, with numerous studies showing effectiveness and safety. However, while Russia licensed vaccines using adenoviral vectors for Middle East Respiratory Syndrome (Mers) and Ebola, there were no large phase 3 studies for them. ‘Neither the adenovirus vectors nor the RNA vaccine have been licensed for any disease [in Western countries],’ notes Ertl. ‘Some have undergone large scale clinical trials, but we have zero experience in mass vaccinations.’
The Russian vaccine uses two different viral vectors, human adenovirus type 26 (Ad26) in an initial dose, and human adenovirus type 5 (Ad5) in a booster three weeks later. Both normally cause common colds, so a proportion of people will already have antibodies to them. That may lessen the vaccine’s effectiveness at stimulating an immune response against the Sars-CoV-2 virus. Ad26 is the same vector being employed by Johnson & Johnson (J&J), while China’s CanSino Biologics is using Ad5.
In principle, Ertl in principle likes the Russian approach of using two vectors. ‘It has a chance of being efficacious, and a reasonable chance of being safe. I just wish they would do a phase 3 trial like everyone else,’ she says. ‘It would take them another two or three months and then I would say great, wonderful job. But this way, I am just shaking my head, like every other scientist in this country.’
If the Russian gamble fails, it could damage public acceptance of other Covid-19 vaccines. ‘If there is a surprise, an adverse reaction which shows up once you inject 20,000 people, then people who wanted the vaccine will change their minds and we will never get a handle on this pandemic, until we have herd immunity in about 20 years,’ says Ertl. Such a failure could also impact acceptance of the vaccines that share the same vectors.
Salmon says he is not worried about European or US regulators pushing the limits in terms of vaccine safety, but has concerns about politicians who ‘don’t always appreciate the value of science’.
I am a freelance science journalist based in Dublin, Ireland. I cover a variety of topics in chemical and biological sciences, as well as science policy, health and innovation.
The deal included an option to purchase 100 million additional doses from the British drugmaker should its vaccine prove safe and effective
The EU said over the past two weeks it was in advanced talks with Johnson & Johnson and Sanofi for their vaccines under development
Brussels: The European Union has agreed to buy at least 300 million doses of AstraZeneca's potential COVID-19 vaccine in its first such advance purchase deal, which could weaken plans led by the World Health Organisation for a global approach.
The European Commission, which is negotiating on behalf of all 27 EU member states, said the deal included an option to purchase 100 million additional doses from the British drugmaker should its vaccine prove safe and effective.
The EU's bilateral deal mirrors moves by the United States and other wealthy states, some of which are critical of the WHO's initiative, and further reduces the potentially available stock in the race to secure effective COVID-19 vaccines.
The EU agreement follows an initial deal with AstraZeneca reached in June by Europe's Inclusive Vaccines Alliance (IVA), a group formed by France, Germany, Italy and the Netherlands to secure vaccine doses for all member states.
The Commission did not disclose the terms of the new deal and declined to say whether it had replaced the IVA's.
"This new agreement will give all EU member states the option to access the vaccine in an equitable manner at no profit during the pandemic," AstraZeneca said in a statement.
The EU executive said its deals are aimed at financing part of the upfront costs to develop vaccines. The funding would be partial down-payments to secure the shots, but actual purchases would be decided at a later stage by each EU state.
The EU said over the past two weeks it was in advanced talks with Johnson & Johnson and Sanofi for their vaccines under development.
It is also in talks with Pfizer , Moderna and CureVac to buy upfront their potential COVID-19 vaccines, EU officials told Reuters in July.
BLOW TO WHO?
The EU move could make more difficult efforts led by the WHO and GAVI, a global alliance for vaccines, to buy shots on behalf of rich and developing countries with a separate scheme.
The Commission has urged EU states to shun the WHO-led initiative because it sees it as too expensive and slow, EU officials told Reuters in July.
Now the Commission is openly saying that vaccines bought from AstraZeneca, and from other vaccine makers, could be donated to poorer states, effectively taking on the very task that the WHO is pursuing with the so-called ACT-Accelerator Hub.
Brussels has publicly said that its purchasing scheme is complementary to the WHO's, but in private told EU states that there may be legal issues if they joined the WHO programme.


No comments:
Post a Comment