By Dr. Liji Thomas, MD Aug 10 2020
The ongoing COVID-19 pandemic is spread by respiratory aerosols, in which tiny droplets of saliva and mucus containing the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are expelled from the upper respiratory tract. Though quite a few studies have investigated the viral load of such droplets, not much is known about how far these droplets move or how long they linger in the air, even though such information is crucial to determining how infectious they are.
Now, a new study by researchers at the University of Twente and University of Rome Tor Vergata and published on the preprint server medRxiv* in August 2020 shows that earlier assumptions about these droplets were wrong. In fact, under 50% relative humidity, the smallest droplets survive 50 times longer, and at 90% relative humidity, up to 150 times longer. In other words, the two-meter or six-foot social distancing rule is grossly inadequate, given the actual advective range of the droplets within one second. And the range, as well as lifetime of the droplet, only increases with smaller droplet size.
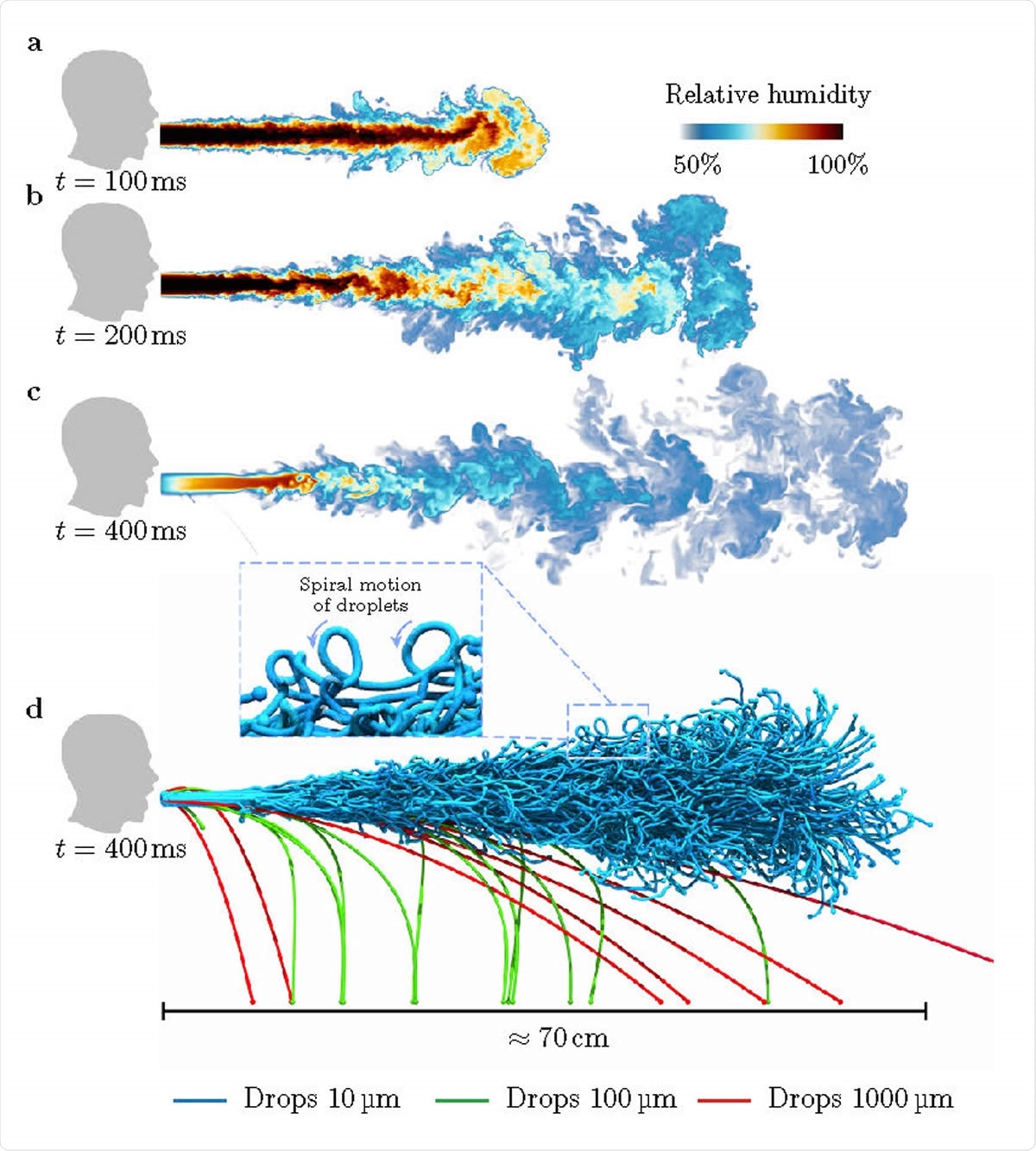
Visualisations of droplets in a heavy cough for RH = 50%: a-d, Snapshots of the droplet-laden cough simulation. At time t = 100 ms, the cough contains hot air with high moisture content. The hot moist air propagates (t ~ 200 ms) and dissipates (t ~ 400 ms) into the ambient surroundings. At t ~ 400 ms, we show larger droplets falling out from the puff whereas smaller droplets remain protected and are carried along by the puff.
Preventing Respiratory Transmission
The current rules of social distancing originated with a 1919 paper dealing with the Spanish flu of that time. This, in turn, was based on a theory of droplet transmission of viruses developed by William F. Wells in connection with the spread of tuberculosis. He thought that the wide range of particles produced by a cough or sneeze in a tuberculosis patient would determine the behavior of the droplets. Small droplets would rapidly evaporate and leave behind less infectious dried aerosol particles with lower transmission risk. Larger droplets would be like bullets. In the current study, droplets measuring over 5-10 micrometers are called respiratory droplets and can cause host-to-host spread. Small droplets, or respiratory droplets, transfer the virus through aerosols.
Despite the age of this principle, the evidence is building that it is faulty. Not only has viral spread continued to take place, especially with superspreaders, but the droplets are known to last longer and spread farther than a few seconds and two meters – namely, up to 8 meters and for up to 10 minutes, respectively. This is because droplets are typically expelled as a cloud, within warm and humid air, which delays their drying out and prolongs their infectious period. In fact, droplet lifetime is dependent on the mixing process within this turbulent air, while the earlier drying behavior is that of a single droplet.
Airborne transmission
This change in fundamental assumptions is supported by empirical studies, medical knowledge, and physics – "long-distance airborne transmission through multiphase turbulent droplet cloud emission is an essential factor." Some researchers have shown that very infectious patients may spread the virus in their aerosols over large distances. In fact, the results of such spread may be even more severe disease due to the tiny droplets of the aerosol, which leads to their entry deep into the lungs.
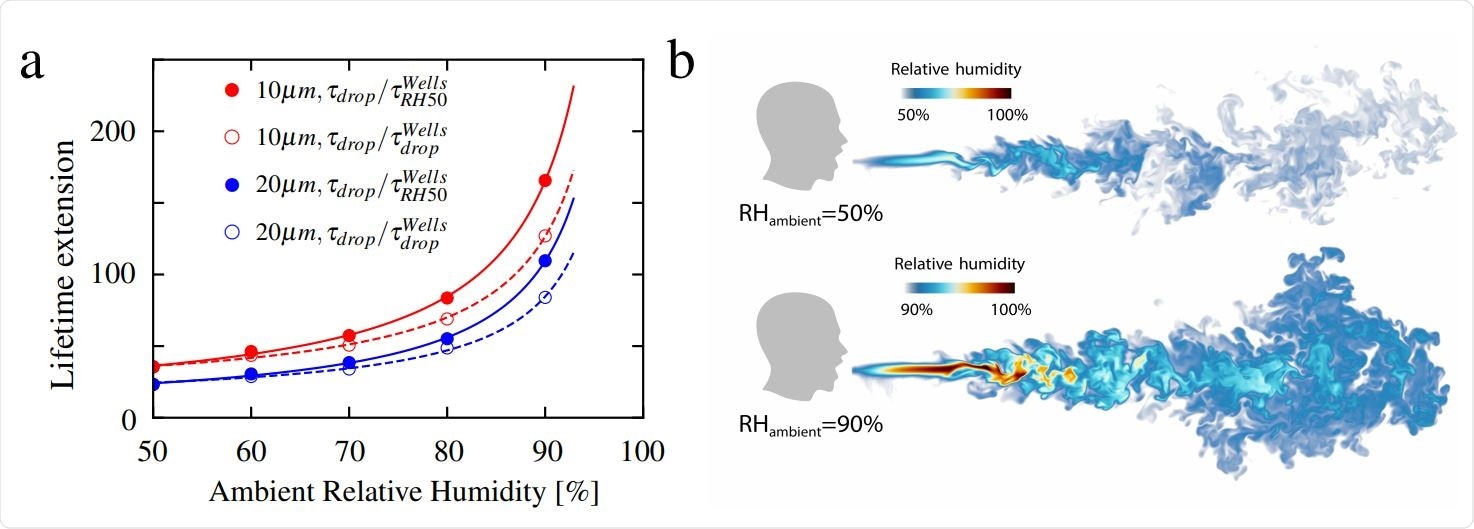
Lifetime ratio for 10 µm and 20 µm droplets: a, Extended lifetime as a function of relative humidity up to RH = 90%. The curves in the figure are fitted according to the function y = a1/(1 − x) + a2, where a1 and a2 are the fitting parameters. Visualisations of humid puff for ambient RH = 50% and 90% at time 600 ms: b, The humid puff maintains coherence for longer time and at much longer distances for larger ambient RH. Note the different humidity color scales for two shown cases.
Humidity and Infectiousness
The current study deals not only with the accumulating nature of aerosols, which remains infectious indoors over hours but also with the little-understood contribution of humidity. Because of the immense difficulty in tracing the movement of thousands of tiny droplets in space and over time, while simultaneously keeping track of or adjusting the conditions such as flow rate, distribution width of the droplets, temperature and relative humidity, the researchers chose to use numerical simulations instead.
Assessing Small Scale Droplet Physics
They tweaked existing methods to ensure that the small scale of the droplet mixing process, as well as the coupling of temperature and humidity, which are so essential to the evaporation of the droplets and thus their lifetime and effects, are properly captured. This involved the development of a very efficient numerical tool that will be of use in revealing the flow physics of an event occurring with breathing, and also what decides the enormous enhancement of the lifetime of a respiratory droplet relative to considering the droplet isolated from its surrounding puff velocity, temperature, and humidity. This tool can also be used to simulate more complicated respiratory events, especially those which take place indoors.
The conditions of the experiment included a duration of 0.6 seconds, simulating a turbulent puff of air into ambient air, full of 5,000 droplets of water, as well as hot air saturated with vapor, in order to replicate a strong cough. The initial temperature was 34 oC. The temperature of the ambient air was set at 20oC, with the relative humidity between 50% and 90%. The heat and vapor in the turbulent puff are exchanged to the ambient air. The researchers tracked the droplets for several seconds to understand the physics underlying their evaporation en masse.
Fall Pattern of Large and Small Particles
The first result at a RH of 50% is the falling out of larger droplets over 100 μm in diameter, in a ballistic manner, because of their weight compared to the airflow, at 0.1 m to 0.7 m from the source. These evaporate faster, compromising the survival of the infectious particles. This agrees with the earliest predictions (Wells, 1930), and the current social distancing guidelines by the World Health Organization (WHO), Center for Disease Prevention and Control (CDC) and the European Centre for Disease Prevention and Control (ECDC).
When smaller than this, however, the droplets form spirals tracing a mostly horizontal path, which means they also promote airborne rather than droplet transmission – unlike the current WHO hypothesis. This is due to their slower settling speed compared to the velocity of the fluid in which they are carried, which means further advection by the turbulent airstream. This latter is essential in airborne transmission of infection.
This small droplet behavior means they have much higher lifetimes than isolated droplets. In fact, 10 μm droplets at RH 50% and 90% have 60 to 200 times the survival times of the Wells value. These move slower related to the fluid flow, and so shrink less due to reduced convection and evaporation.
With successive coughs, therefore, the puff may reach over 2 m from the source at the leading edge, with most of the smaller droplets being in humid surroundings and thus living longer.
Implications and Recommendations
Thus, the study shows that the humidity field around the droplet plus the turbulent velocity, and not just the droplet diameter, determines the respiratory droplet lifetime. This boosts their lifetime by orders of magnitude. The ambient RH further extends the lifetime, and the researchers comment, "This finding may explain why many COVID-19 superspreading events have been reported in indoor environments with large ambient relative humidity." They quote the high spread in meat-processing plants with cooled air, which increases the indoor RH immensely.
This means that aerosol and droplet concentration must be controlled indoors, especially in the coming fall and winter. Again, older medical experts like Soper (1919) are proved right with their claim, originally pertaining to the Spanish flu pandemic of those years, that "there is danger in the air in which they cough and sneeze." Even further, according to the current study, "we must also add "speak," "sing," scream," and even "breath." In fact, Soper recommended open windows at home and work, and masks for suspected patients – an excellent protocol for today at well.
The current study thus confirms and explains Soper's mitigation strategies for use in controlling COVID-19 transmission. The researchers say face masks block respiratory droplets indoors, and some may even reduce the inhalation of these droplets, an essential role for healthcare workers in the pandemic.
Excellent ventilation is equally important to ensure the infectious puff advects out of the room or becomes rapidly and highly diluted. A possible unfavorable effect of this is that good ventilation may increase the length of the propagation path of the droplets, and these two effects of ventilation on transmission properties must be investigated in parallel.
Finally, a lower ambient RH will help speed up the evaporation of the droplets and aerosols, reducing infectivity by decreasing the lifetime of infectious particles and aerosols.
The study sums up, "Our results help to understand why these various mitigation strategies against COVID-19 are successful…. Our present tool and approach will be a starting point for larger parameter studies and for further optimizing mitigation strategies ."
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
Chong, K. L. et al. (2020). Extended Lifetime of Respiratory Droplets in A Turbulent Vapour Puff And Its Implications On Airborne Disease Transmission. medRxiv preprint doi: https://www.medrxiv.org/content/10.1101/2020.08.04.20168468v1






_Bio.jpg)
_Bio.jpg)
_Bio.jpg)




