How tadpoles provide insight into pandemics
by Sara Putnam, University of Connecticut

A virus affecting wood frog tadpoles throughout the eastern United States is offering scientists a rare opportunity to investigate the role of environmental factors in the spread of infectious disease.
An important aspect of controlling the spread of any virus is understanding how the virus, or agent, is transmitted through the environment to the host. Scientists refer to the trio of agent, host, and environment as the epidemiological triangle or triad. In the COVID-19 pandemic, the agent is the SARS-CoV-2 virus, humans are the host species, and the environment now includes ecosystems throughout the planet. Scientists have made strides in understanding the nature of the SARS-CoV-2 virus and the infection it causes in its human hosts, and they have identified variables in the host population that can cause some individuals to be more severely affected by the virus than others. As researchers continue to learn about environmental factors that facilitate the spread of COVID-19, we practice social distancing and wear masks to contain airborne droplets from our mouths and noses.
The interrelationships among agent, host, and environment are complex, and possible variables in each of the three are limitless, making it impossible, when studying them as a system, to tease out the differential effects of the individual players. While agent and host can be studied in isolation and in direct relationship to each other within the laboratory, environmental factors also play a role in disease dynamics, meaning that any conclusions reached will not fully reflect that happens in natural systems. However, Associate Professor Tracy Rittenhouse has developed an experimental model where the focus is the effects of the environment in epidemics rather than the details of the agent and host interaction.
Several years ago, Rittenhouse, a faculty member in the Department of Natural Resources and the Environment, learned of wood frog tadpole dieoffs in northeastern Connecticut that had been found to be caused by Ranavirus Frog virus 3 (FV3). To measure the prevalence of FV3 in the area, Rittenhouse harvested tadpoles from numerous wetlands over a two-year period and found that the frog virus was much more widespread than previously known. But, the population of wood frogs, a species known to be particularly susceptible to FV3, did not appear to be declining, and some live tadpoles harvested from wetlands were found to be infected with the virus, both of which indicated that the virus was not always lethal.
From her surveys, Rittenhouse learned that when tadpole dieoffs did occur, it was often at the same developmental stage, just before metamorphosis. The carcasses would remain visible in the water for only one to three days. The tadpole carcasses decomposed so quickly it was if they had disappeared. Determining whether tadpoles died and decomposed or metamorphosed into frogs and left the wetland would have required simultaneously monitoring all wetlands where the virus was known to be present, which was not feasible.
Says Rittenhouse, "We don't have a good explanation for why we could have found the virus so commonly and not have dieoff events. I think there's some middle ground. I believe there are more dieoff events happening than we're detecting, but we don't yet know what triggers them. We're concerned that changes occurring in environmental conditions, such as salinity levels or temperature, might be increasing the likelihood of dieoffs." An additional question is whether the tadpoles are particularly susceptible at the stage when dieoffs have occurred, or if something occurs in the environment at that time.
A fruitful collaboration
The study of interactions among agent, host, and environment is best done with a collaborative approach incorporating a range of expertise. Says Rittenhouse, "I study populations. I know a lot about wood frogs–I know where wood frogs live, what types of environments they live in, what causes high survival, and what causes low survival. But I'm not a disease expert." So, she teamed up with Jesse Brunner, a disease ecologist, and Erica Crespi, a physiologist, both at Washington State University, who have studied Ranavirus and its effects on individual tadpoles. Brunner specializes in the relationship between Ranavirus and its host, while Crespi is an expert on tadpole health.
Rittenhouse devised a set of experiments in which two environmental stress factors—salinity and temperature—are manipulated to identify what, if any role, they play in triggering dieoffs related to FV3. Says Rittenhouse, "Road salt and temperature are two environmental conditions that we're manipulating because they're common things in the environment, and they are both changing a lot, regionally and globally. Salinization of our fresh waters is a very hot topic because it's happening along our coastlines. As sea level rises, there's saltwater intrusion into freshwater systems and into terrestrial environments. But that's also happening in forests in terrestrial conditions when we add road salt to our roads, and it runs off into freshwater wetlands and streams."
During the spring of 2019, Rittenhouse built outdoor experimental systems called mesocosms, which simulate the natural environment but allow for the control of some factors. She set up 150 fifty-gallon tanks, each of which, when filled with water, leaf litter and tadpoles, represents a wetland. These wetlands received natural rainfall, nutrient inputs from the air (think oak tree pollen in the spring), and daily temperature fluctuations as the sun rose and set. She controlled for variables in the virus and host: Egg masses from different wetlands were mixed to create heterogeneous but similar populations for each tank, and Brunner isolated and extracted the virus from samples Rittenhouse collected in the wild. Finally, one tadpole infected with FV3 was added to each tank. In her experiments, Rittenhouse manipulated the temperature and salinity levels of some tanks and maintained some tanks as controls.
Rittenhouse, Brunner, and Crespi had developed hypotheses for expected mortality rates in response to the environmental manipulations. As the tadpoles matured, Rittenhouse and the students in her lab group monitored all the tadpoles in every tank, every day during May and June 2019. The result was more than twenty epidemiological curves for each temperature-salt combination.
"What our project brings is the ability to manipulate a population and see how changes to environmental conditions change how spiked or flat an epidemic curve is," Rittenhouse says. "And there really are not a lot of study systems where you can manipulate that epidemic curve. Much of what we know about disease epidemics is based on mathematical models. Our project uses a study system where we can manipulate a population and quantify an epidemic curve in a two-to-three-month period for 150 populations, but it's real data from real animals. It's a way to confirm that some of our mathematical models are correct."
Ironically, the studies Rittenhouse planned for continuing the FV3 research during the summer of 2020 had to be postponed due to the COVID-19 pandemic. The experiments would have required eight undergraduate student research assistants working together every day to monitor tadpole mortality. So, like everyone else, Rittenhouse pivoted. No frog virus was put into the tanks. Instead she and one graduate student focused their efforts on finely tuned manipulations of salt and temperature in the absence of the virus.
Next spring, when more is known about how people can work safely in a world with COVID, then Rittenhouse and her students will return to her NSF- funded research on Ranavirus epidemics.
Rittenhouse says, "Each spring in my population dynamics course, I teach students how to use data we collect in the wild, counting animals, to develop estimates of birth rates, death rates, survival rates. We take those estimates and build population-level models that predict if a population is going to increase or decrease over time and link that to trends we observe in the wild. But in this case, the cool thing is we can create all these experimental populations—150 different populations—and we can measure the population response. How peaked was the curve? How flat was the curve? What's the timespan between the beginning and end of the epidemic? How do changes in environmental conditions that might be stressful for individuals or populations change the shape of an epidemic curve?"
Explore further
Provided by University of Connecticut


 The density of red foxes is increasing in Norway’s mountainous areas. The more trash and food waste red foxes have access to, the greater their numbers. This photo was taken with a game camera and shows a red fox that has found food. Credit: NINA, game camera
The density of red foxes is increasing in Norway’s mountainous areas. The more trash and food waste red foxes have access to, the greater their numbers. This photo was taken with a game camera and shows a red fox that has found food. Credit: NINA, game camera Food waste left along the road at Dovre – just the kind of items that attract scavengers. Credit: Lars Rød-Eriksen
Food waste left along the road at Dovre – just the kind of items that attract scavengers. Credit: Lars Rød-Eriksen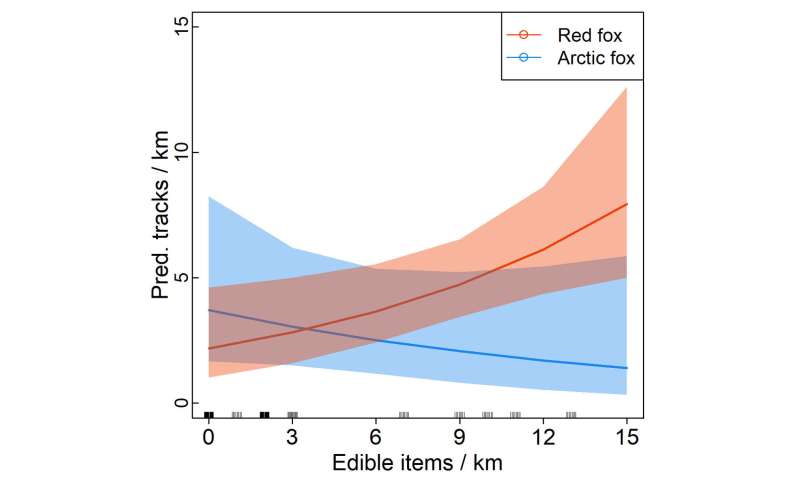 The graph shows that the number of red fox tracks per kilometre (y-axis) increases with the amount of edible waste (x-axis).
The graph shows that the number of red fox tracks per kilometre (y-axis) increases with the amount of edible waste (x-axis). A lot of trash means few Arctic foxes. We found that the Arctic fox doesn’t tend to stay close to the road – probably not because it isn’t attracted to the road, but because the red fox’s presence makes it stay away, the researcher says. The photo was taken in Lesja municipality. Credit: NINA, game camera
A lot of trash means few Arctic foxes. We found that the Arctic fox doesn’t tend to stay close to the road – probably not because it isn’t attracted to the road, but because the red fox’s presence makes it stay away, the researcher says. The photo was taken in Lesja municipality. Credit: NINA, game camera