The lowdown on hydrogen – part 2: production
 Electrolysis, powered by renewables, is often seen as the ideal way to produce hydrogen. But electrolysis is expensive and not always efficient, writes Roger Arnold. There are other ways that are more efficient and also climate friendly. This is part 2 of a two-part series on hydrogen written by independent energy expert Roger Arnold. Part 1 deals with the uses of hydrogen in transport.
Electrolysis, powered by renewables, is often seen as the ideal way to produce hydrogen. But electrolysis is expensive and not always efficient, writes Roger Arnold. There are other ways that are more efficient and also climate friendly. This is part 2 of a two-part series on hydrogen written by independent energy expert Roger Arnold. Part 1 deals with the uses of hydrogen in transport.
In part 1 last week, I reviewed current status and issues surrounding battery vs. fuel cell electric vehicles. There’s more to be said about that, but not just now. My overall aim here is to explore and clarify — for myself as much as anyone — issues around electricity and hydrogen in a sustainable clean energy economy. In particular, I’d like to understand the significance of the apparent resurgence of interest in fuel cells and the “hydrogen economy”. Toward that end, I think the next order of business is to look at options for hydrogen production. There are new possibilities that I only recently learned about that may prove significant.
What’s wrong with electrolysis?
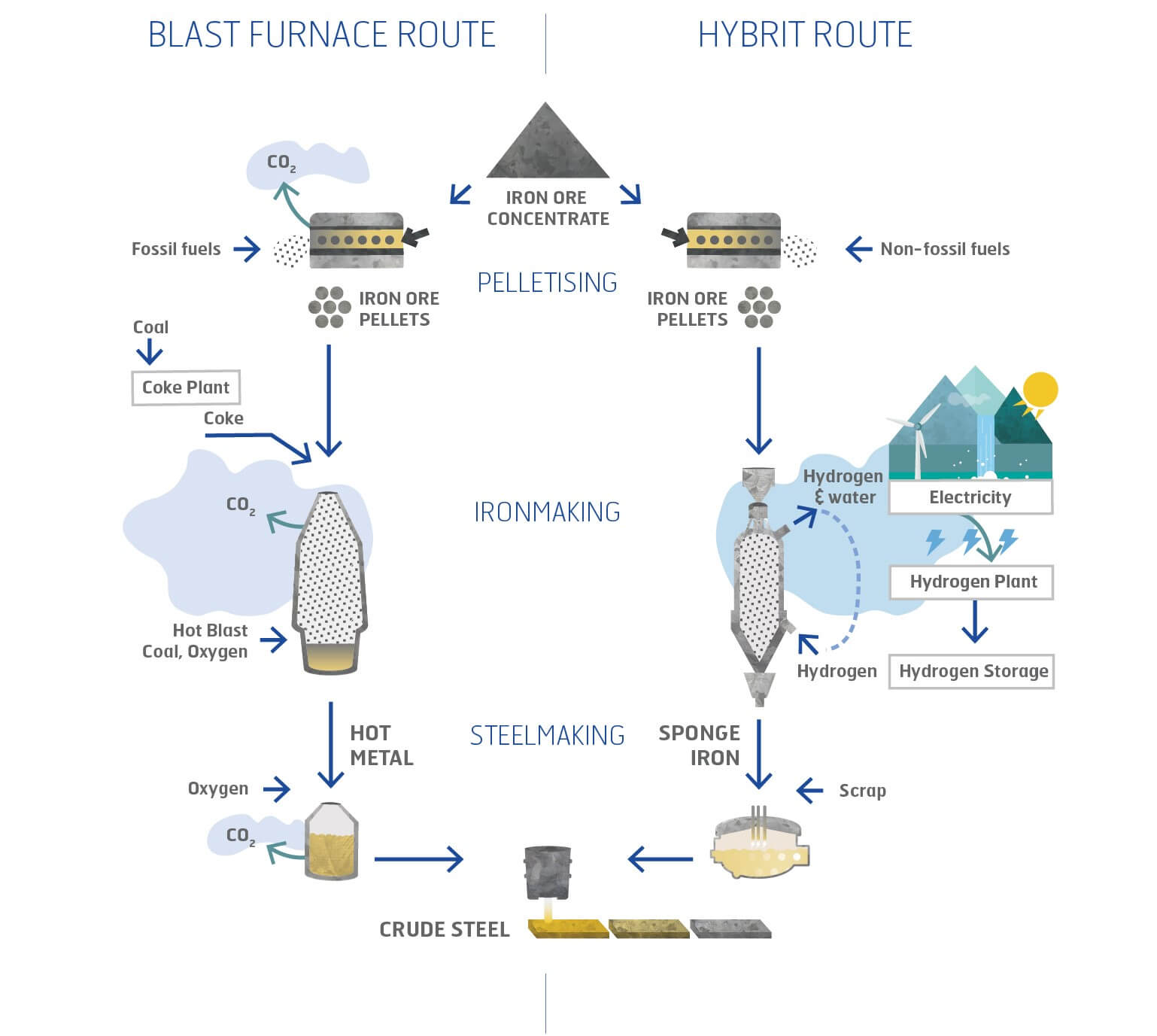
There are many ways to produce hydrogen. There are only two, however, that are commercially significant in the US and most other nations. One is steam methane reforming (SMR), which starts with natural gas as feedstock. The other is electrolysis of water.
Inputs for electrolysis are just electricity and water. If the electricity is from carbon-free renewable resources, the resulting hydrogen is also carbon-free. That makes electrolysis very attractive to renewable energy advocates. It’s seen as a way to make use of excess power and stabilize the grid when wind and solar resources are making more power than the grid can otherwise absorb.
The larger issue for renewable energy is undersupply, not oversupply
It can certainly do that. And producing hydrogen by electrolysis is more valuable than curtailing power production or dissipating it in a resistor bank when there is truly excess supply. However the larger issue for renewable energy is undersupply, not oversupply. When the sun isn’t shining and the wind isn’t blowing, there’s still demand to be met. There needs to be a way to meet it. If it’s not dispatched fossil fuels, it has to be energy drawn from storage.
Hydrogen does in fact make it feasible to store enough energy to meet demand during lulls. Even extended lulls. The problem is that it’s inherently expensive. That matters, because solutions that cost more don’t get adopted when there are cheaper alternatives available. And dispatching fossil fuel generators is a cheaper alternative that is definitely available.
So why is electrolytic hydrogen inherently expensive? If the electricity driving the electrolysis is surplus, to the point that the alternative to electrolysis is curtailment, the cost of that electricity should be almost nothing. Won’t the hydrogen produced also be very cheap?
Well, it would be, if the capital cost of the electrolysis equipment were negligible, or if renewable energy available for “almost nothing” were common enough to power the equipment at a decent duty cycle. Neither of those conditions applies, however.
The fraction of delivered power that stored hydrogen would need to supply depends strongly on what other resources are online and what level of demand side management has been implemented. In the conceptually appealing but economically worst case scenario of 100% wind and solar, no demand side management, and no super transmission systems, energy from stored hydrogen would represent about three quarters of all kilowatt-hours delivered.
In any system where a substantial fraction of delivered kilowatt-hours must come from storage, the very poor round-trip energy efficiency of power-to-gas-to-power bites hard
That’s of course not very realistic, since even a 100% renewables scenario will include a degree of dispatchable hydroelectric, some demand side management, and a transmission system sufficient to provide partial statistical leveling of wind and solar availability. Even so, with no baseload supply and no resort to dispatched fossil generation, stored hydrogen would need to provide at least a quarter of kilowatt-hours delivered in most cities below 40 degrees latitude. At higher latitudes, seasonal variation in solar availability would raise the figure.
In any system where a substantial fraction of delivered kilowatt-hours must come from storage, the very poor round-trip energy efficiency of power-to-gas-to-power bites hard. 40% is usually taken as the highest round-trip efficiency one could expect for a PEM electrolyzer – fuel cell combination.
If the gas-to-power side employs simple gas combustion turbines rather than fuel cells — as might be the case for generating occasional high power output from a central facility near a gas storage cavern — the round trip efficiency could easily be under 30%. But even at an optimistic 40%, 2.5 kilowatt-hours of input energy would be needed for every kilowatt-hour of energy delivered from storage.
If energy delivered from storage were 25% of energy consumed, then total energy produced would need to increase by three eighths (37.5%) to cover round trip energy losses for that 25%. Renewable energy production at 137.5% of total load would divide as 75% to direct service of load and 62.5% dedicated to hydrogen production for energy storage.
The numbers get rapidly worse if one assumes less than 40% round trip efficiency or greater than 25% of load supplied from stored energy. That doesn’t actually matter to the bottom line, however. The bottom line is that if coping with intermittency of supply in a 100% renewable energy economy were to be based on electrolysis of water, electrolysis would represent by far the largest load on the system. It could not run on “almost free” surplus energy, because there would be none.
The electrolysis load would consume every bit of power output not required by higher priority loads, and still need more. It could go offline at times of shortage, thus avoiding peak charges. But it could never get by on “almost free” surplus power. It would have to pay at the full rate needed to amortize the capital and operating expenses of the renewable energy systems dedicated to feeding it.
Prospects for improvement?
What about prospects for better efficiency in the future? The theoretical potential is always there, but near-term practical prospects look poor. A good part of the inefficiency that besets water electrolyzers and hydrogen fuel cells is “semi-fundamental”. It’s rooted in the substantial overpotential associated with the oxygen evolution reaction (in electrolyzers) and its inverse (in fuel cells).
The overpotential for the oxygen reactions is analogous to the forward voltage drop across a P-N diode junction. At any positive forward bias, the P-N diode junction should theoretically conduct some current. And in fact one can observe that it does — given a sufficiently sensitive lab setup. The current is exponential with voltage, but the knee of the curve doesn’t show up until the forward bias rises above about 0.6 to 0.7 volts.
Historically, the cost of hydrogen from SMR has averaged roughly a quarter to a third of the cost of hydrogen from electrolysis
In a similar manner, an electrolysis cell will evolve tiny amounts of hydrogen and oxygen at any cell voltage above the 1.23 volt equilibrium voltage of water at ambient temperature. But the amounts will be too tiny to notice until the cell voltage rises above about 1.7 volts. Conversely, a hydrogen fuel cell won’t produce a noticeable current against a cell voltage higher than about 0.8 volts.
The required overpotential can be reduced considerably at high temperatures — meaning in this case upwards of 500 ℃. “Steam electrolysis”, a cousin to solid oxide fuel cell technology, has long been of interest as a more efficient way to split water. But it’s never been successfully commercialized. The problem has been poor durability of the ceramic electrolyte in a hot hydrogen environment. I’ve seen no indications of that being about to change.
Steam methane reforming
If hydrogen can’t be produced cheaply by electrolysis in quantities large enough to be useful for grid-scale energy storage — much less for a major transportation fuel — what about other production methods? What about steam methane reforming?
The cost of hydrogen from SMR is linked to the cost of natural gas. But so, increasingly, is the cost of electricity. Historically, the cost of hydrogen from SMR has averaged roughly a quarter to a third of the cost of hydrogen from electrolysis. That’s in line with what one might expect, given that it takes nearly three units of natural gas, on average, to produce one unit of electricity, while the energy efficiency of electricity to hydrogen is worse than that of natural gas to hydrogen.
With that much of a cost difference, it’s no surprise that SMR supplies 95% of the market for hydrogen used in oil refining and other industries. Hydrogen from electrolysis is only used when the quantities needed are small or the purity requirements exceptionally high.
The downside of hydrogen from SMR is that natural gas is a fossil fuel, and the prevailing methods of implementing SMR vent fossil carbon into the atmosphere. That’s because the primary reaction:
CH₄ + H₂O + heat ⇌ CO + 3H₂
is strongly endothermic; it requires heat to drive it forward. That heat is usually supplied by burning some of the natural gas, producing CO₂. The CO in the output stream from the primary reaction is usually also converted to CO₂ via the water gas shift reaction:
CO + H₂O ⇌ CO₂ + H₂ + heat
There are many ways to engineer the reaction train for SMR. In most cases a waste stream of CO₂ and N₂ (from the air used in burning some of the gas) is produced. The mixture is usually vented. However, in situations where a pure CO₂ stream has value, it can be arranged at little added cost. Either the CO₂ can be separated out, or the reaction train can be engineered so that atmospheric N₂ never enters the gas flow in the first place.
There is an existing market for CO₂ that is already fairly large. If it develops as many expect over the next few decades, it will be large enough to absorb the CO₂ supply from even large scale production of hydrogen by SMR
A good technical presentation from the Colorado School of Mines covers several variations for SMR trains. It’s not exhaustive; there are significant variations that it doesn’t cover, including a new one recently announced that promises to make clean SMR with a pure CO₂ waste stream practical at the scale of a hydrogen refueling station. The capital cost would be low. The station would need to be located near a CO₂ pipeline to facilitate disposal of the CO₂, but that’s not impossible.
The low cost of producing hydrogen from natural gas, along with the relative ease of producing a “pipeline ready” CO₂ waste stream, mean that carbon-free hydrogen could in fact be supplied to fuel cell vehicles at a cost below the equivalent amount of gasoline.
Disposal of CO₂
If we should use SMR to produce cheap hydrogen for FCVs, how will we dispose of the CO₂? And how will disposal affect the cost of the hydrogen produced? It’s a non-trivial question.
Given the slow turnover rate in the automotive vehicle fleet, it can take a decade or two for even the most favorable new technologies to permeate the fleet. In view of that, the amount of CO₂ associated with hydrogen production for FCVs is unlikely to become significant any time soon. There are commercial uses for small quantities of CO₂ that are not controversial. That market is probably large enough to absorb the supply of CO₂ from hydrogen production for the automotive market for at least the next ten years. Beyond that — and especially if hydrogen is adopted for backing renewables on the electricity grid — larger markets for CO₂ will have to be tapped.
There is an existing market for CO₂ that is already fairly large. If it develops as many expect over the next few decades, it will be large enough to absorb the CO₂ supply from even large scale production of hydrogen by SMR. But it’s not without controversy. It’s the market for CO₂ based Enhanced Oil Recovery (EOR).
CO₂ based EOR involves pumping of compressed CO₂ through injection wells to an oil-bearing formation. It restores pressure in the formation and forces remaining oil toward production wells. It also mixes with the oil, expanding its volume and reducing its viscosity. That enables it to flow more easily through the porous rock of the oil reservoir. As explained in this document from the Global CCS Institute, injection of CO₂ into mature oil fields is increasingly considered the most effective method available to revive output and keep the fields producing.
With the oil age drawing to a necessary close, it makes sense to capitalize on the desire of incumbent producers to maintain their positions for as long as demand holds. By getting the most out of old wells, we can undercut the market for new wells
The controversy around CO₂ based EOR is at multiple levels. It recovers oil from mature fields that would not otherwise be recoverable. Many object to it on that basis alone. They feel that oil should be left in the ground, and that any technology that allows more of it to be recovered must to opposed. But that position rests on an implicit assumption that we will ultimately burn all the oil that is recoverable, and that technology to increase what can be recovered will increase what will be released into the atmosphere.
If we expect to leave a habitable world for future generations, we will have to stop well short of burning all the oil that is recoverable. But in that case, it doesn’t ultimately matter if technology makes more of the oil from mature oil fields recoverable. That only affects who will produce whatever oil we end up burning, and where it will be produced. We’ll stop before it’s all gone, because we have to.
In that case, with the oil age drawing to a necessary close, it makes sense to capitalize on the desire of incumbent producers to maintain their positions for as long as demand holds. By getting the most out of old wells, we can undercut the market for new wells while disposing of large amounts of CO₂ in the bargain.
Other objections to CO₂ based EOR have to do variously with doubts about safety, duration of sequestration, and economic viability. Those are large topics, and I won’t try to address them in any detail in this post. However, CO₂ based EOR is not a new thing, and oil and gas industry has decades of experience with it on which to draw.
In fact, there are already some 3600 miles of pipeline in North America for transporting CO₂ from various sources to EOR sites. The map below, from an NETL review of CO₂ pipeline infrastructure in the US, shows the pipelines and CO₂ sources as of 2014, and the oil field regions served.
Notably absent are the oil fields of Kern County and southern California. The nature of those fields makes them good candidates for CO₂ EOR, but there is no large natural CO₂ source nearby that would make a pipeline network to the fields attractive to investors. There are certainly many industrial facilities that could be equipped for carbon capture. If a CO₂ pipeline ran near them, they could connect to it and profit from sale of their captured CO₂. However, none has been large enough to anchor construction of a pipeline. So it hasn’t happened.
Byproduct carbon black from methane cracking could become the ultimate stored energy resource. Stores of terawatt-hours of energy could easily be accumulated
It’s possible that the State of California, if it came to see SMR with carbon capture as the most practical route to success of zero-emission hydrogen FCVs, might do something about that. It could offer loan guarantees for construction of the pipeline, along with the SMR plants that would feed it. Disposal of CO₂ would then reduce, not increase, the cost of hydrogen fueling stations that the state wants to see. If I were an executive at Toyota, Honda, or Hyundai, I’d be enrolling oil field operators and directing lobbyists in Sacramento to promote that idea.
Beyond SMR
There might be another way to produce hydrogen from natural gas that would obviate the need for disposal of CO₂. That’s by cracking methane (and other volatile hydrocarbons in natural gas) to produce pure carbon and hydrogen. It’s a high temperature reaction that is very familiar to chemical engineers. It’s even used commercially to produce carbon black for use in tires and other rubber products. But the method used to drive the reaction has been a plasma arc. That approach is extremely energy intensive.
A few research groups have been exploring other approaches that would be much more efficient. The most promising alternative may be one that was reported in the cover article in an August 2016 issue of New Scientist. It was dramatically titled “The Reaction that Will Change the World”. It involves bubbling natural gas through molten tin at ~1000 ℃. Carbon from the natural gas splits from its hydrogen atoms and dissolves into the tin. The orphaned hydrogen bubbles to the top of the pool, where it’s separated from any unreacted methane through a hydrogen permeable membrane. The unreacted methane is recycled. The dissolved carbon, meanwhile, precipitates as microparticles of carbon black that migrate to the surface of the pool. Accumulated carbon black can be scraped from pool of tin in the same manner as slag from a pool of molten steel.
The process only produces half as much hydrogen per unit of natural gas as SMR, but it does so with high energy efficiency. There is no CO₂ needing to be transported by pipeline and pumped into geological storage reservoirs far underground. The waste stream is fine carbon black that has many potential uses. High value uses would be in water and air filters (in place of activated charcoal) and as filler material in tires and in rubber and plastic products.
It does seem to me that the idea of clean hydrogen from chemical processing of fossil fuels offers game-changing potential
If implemented to supply hydrogen on a large scale, the amount of byproduct carbon black produced from cracking methane would saturate the high use value markets. The rest might be sold as a soil amendment with properties similar to biochar — although its efficacy for that would need to be proven. However, there’s another scalable use that might be feasible: it might be used to fuel flexible power generation from direct carbon fuel cells.
Direct carbon fuel cells of various designs have been demonstrated, and there are ongoing efforts to commercialize them. Some approaches have shown efficiencies as high as 80% for electricity out to chemical potential energy in. But efforts have almost all been funded by DOE as part of its “clean coal” initiative. They’ve accordingly been directed at making the cells work using powdered coal as fuel. The impurities in coal have made that difficult. It’s possible that with a highly pure form of carbon black as fuel, commercial feasibility would be much easier to reach. If so, byproduct carbon black from methane cracking could become the ultimate stored energy resource. Stores of terawatt-hours of energy could easily be accumulated.
Of course, actually using carbon black as fuel for direct carbon fuel cells (DCFC) reintroduces the sequestration issue for the resulting CO₂ waste. But it’s a pure CO₂ waste stream, and the ease of transporting bulk carbon black means that the DCFC plants could be located close to CO₂ injection sites. If the DCFC approach works, it would be a way of shortcutting the CO₂ pipeline infrastructure problem.
Backing renewables
Regardless of whether methane cracking and possibly the DCFC approach to massive stored energy work out, it does seem to me that the idea of clean hydrogen from chemical processing of fossil fuels offers game-changing potential. The carbon or CO₂ waste streams can be sequestered at little cost — or negative cost in the case of CO₂ sold for EOR. That means that the hydrogen produced can truly be zero carbon, even before the power grid is decarbonized.
Most compelling, to me, is that $53 per kilowatt figure I mentioned last week as DOE’s estimate for the current system cost for PEM fuel cell stacks (in volume production). I’ve long held that the biggest problem with intermittent renewables is their effect on backing supplies. Under high penetration scenarios, backing supplies see short operating periods, fast ramp rates, and low overall duty cycles. That forces utilities to resort to the cheapest units they have to fill the role. Those are mostly simple combustion turbines that have high carbon emissions. However, $53 per kilowatt is even cheaper than simple combustion turbines. And PEM fuel cell stacks don’t care about operating periods or ramp rates. Their thermal efficiency compares to the best CCGTs, and doesn’t suffer from operation at partial capacity. They’re ideal!
The only problem is supplying them with enough hydrogen fuel. Electrolysis, as already noted, is too inefficient and too expensive. But when surplus RE is used to drive the endothermic SMR or methane cracking reactions, it yields more than a factor of ten more hydrogen than it would by electrolysis. One still has zero carbon fuel being made from cheap “as available” power, and thus the benefit of a “virtual battery” via control of power directed to the process. However, the degree of excess RE capacity needed to supply the fuel is drastically reduced.
I can imagine a whole host of issues and objections that are likely to be raised in response to my conclusion. Rather than trying to anticipate and address them all here, I think I’ll let them play out in the comments. Perhaps I’ll end up posting a subsequent column on the political and environmental issues around “zero carbon hydrogen” from fossil fuels. It will be a hot topic.
Editor’s note
Roger Arnold, systems architect at Silverthorn Engineering, is a former software engineer. He studied physics, math, and chemistry at Michigan State University’s Honors College , where he graduated in 1967. A US Army veteran (courtesy of the Viet Nam era draft), he later did graduate work in computer architectures and operating systems at the University of Colorado. Over the years, he has worked variously for IBM, Boeing Aerospace, AT&T, and about a dozen smaller companies and startups. Since retiring from professional life as a processor architect, he has refocused on clean energy technologies, energy efficiency, and space systems. His favorite activities are currently technical writing and mentoring early stage startups.
This article was first published on Energy Post’s sister publication The Energy Collective and is republished here with permission from the author.