November 10, 2021
Sponges are simple multicellular organisms, though they are skillful filter feeders, filtering tens of thousands of liters of water through their bodies each day to get food. Their ability for this complicated behavior is more exceptional as they do not have a brain or a single neuron.
According to a study published in Science, sponges use a complex cell communication system to regulate their feeding and potentially annihilate invading bacteria. This is exciting research that enables us to look at sponges in a whole new way. According to Casey Dunn, an evolutionary biologist at Yale University, Connecticut, the results could benefit us in understanding the evolution of animals’ nervous systems.
 This freshwater sponge (Spongilla lacustris) may hold clues about the evolution of the nervous system. Image Credits: Willem Kolvoort/Nature Picture Library
This freshwater sponge (Spongilla lacustris) may hold clues about the evolution of the nervous system. Image Credits: Willem Kolvoort/Nature Picture LibraryNeurons interact with one another by transferring electrical or chemical signals via small and targeted connections termed synapses. Despite the animals’ lack of neurons, earlier research discovered that sponges have genes encoding proteins that support synapses function.
Detlev Arendt, an evolutionary biologist, EMBL, Germany, and team sequenced the RNA in several separate cells from a freshwater sponge (Spongilla lacustris) to
determine which cells expressed these genes.
They observed that the sponge has 18 distinguished cell types. Synaptic genes were active in some of them, which were grouped near the digestive cells of the sponges. This implies that some kind of cellular interaction may coordinate the animal’s filter-feeding behavior.
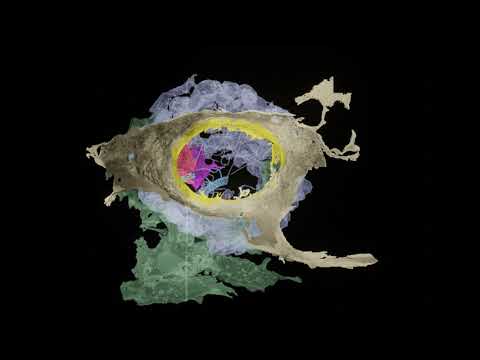
The team then studied one of these cell types, which they named secretory neuroid cells, using X-ray imaging and electron microscopy. The study uncovered that neuroids extend long arms to reach choanocytes, a type of cell with hair-like projections that power sponges’ water-flow systems and capture the majority of their food.
Nervous-system precursor
The team believes that these arms enable neuroids to interact with choanocytes, allowing them to halt the water-flow system and clean out any detritus or foreign microorganisms, based on the proximity of the two cell types and the expression of genes that may allow for chemical secretion. Though these neuroid cells are not nerves, and there is no evidence of the synapses that allow neurons to interact so fast.
Jacob Musser, an evolutionary biologist, EMBL, and co-author of the study, stated that this cell type could be an evolutionary precursor to an actual nervous system.
He further added that they are at an intermediary position, where they have gone from having all these independent pieces to bringing them together more extensively, yet all the interconnectivity required to form a fast synapse is not obtained.
Some experts believe that referring to these cells as nervous system precursors is a stretch. Linda Holland, an evolutionary developmental biologist, UC San Diego, stated that it’s intriguing but not conclusive. She commented that it would be challenging to determine whether the evolution of nervous systems happened from this cellular communication system or arose earlier or even several times, as suggested by some experts. According to Sally Leys, a marine biologist, the University of Alberta, Canada, several other organisms, like unicellular eukaryotes, have the same synaptic genes.
April Hill, a developmental geneticist, Bates College, Maine, believes that this research and its methods will serve as a “launchpad” for further studies into this ubiquitous sponge. She further stated that whether other sponges utilize a comparable cellular interaction system is an important unanswered mystery.
What sponges can tell us about the evolution of the brain

Despite its central importance, the brain's origins have not yet been uncovered. The first animal brains appeared hundreds of millions of years ago. Today, only the most primitive animal species, such as aquatic sponges, lack brains. Paradoxically, these species may hold the key to unlock the mystery of how neurons and brains first evolved.
Individual neurons in a brain communicate via synapses. These connections between cells lie at the heart of brain function and are regulated by a number of different genes. Sponges do not have these synapses, but their genome still encodes many of the synaptic genes. EMBL scientists asked the question why this might be the case. Their latest findings are published today in the journal Science.
"We know that these synaptic genes are involved in neuronal function in higher animals. Finding them in primitive species like sponges begs the question: if these animals don't have brains, what is the role of these genes?" said Detlev Arendt, EMBL group leader and senior scientist at EMBL Heidelberg. "As simple as that sounds, answering this question was beyond our technological abilities so far."
To study the role of these synaptic genes in sponges, the Arendt lab established microfluidic and genomic technologies in the freshwater sponge Spongilla lacustris. Using these techniques, the scientists captured individual cells from several sponges inside microfluidic droplets and then profiled each cell's genetic activity.
"We showed that certain cells in the sponge digestive chambers activate the synaptic genes. So even in a primitive animal lacking synapses, the synaptic genes are active in specific parts of its body," said Jacob Musser, research scientist in the Arendt group and lead author on the study.
Sponges use their digestive chambers to filter out food from the water and interact with environmental microbes. To understand what the cells expressing synaptic genes do, the Arendt group joined forces with six EMBL teams as well as collaborators in Europe and worldwide. Working with EMBL's Electron Microscopy Core Facility, Yannick Schwab's team and Thomas Schneider's group operating synchrotron beamlines at EMBL Hamburg the researchers developed a new correlative imaging approach. "By combining electron microscopy with X-ray imaging on a synchrotron beamline we were able to visualize the stunning behavior of these cells," Dr. Schwab explained.
The scientists captured three-dimensional snapshots of cells crawling throughout the digestive chamber to clear out bacterial invaders and sending out long arms that enwrap the feeding apparatus of specific digestive cells. This behavior creates an interface for targeted cell-cell communication, as it also happens across synapses between neuronal cells in our brains.
"Our results point to the cells regulating feeding and controlling the microbial environment as possible evolutionary precursors for the first animal brains," Dr. Musser said.Microglia pruning brain synapses captured on film for the first time
More information: Jacob Musser et al, Profiling cellular diversity in sponges informs animal cell type and nervous system evolution, Science (2021). DOI: 10.1126/science.abj2949. www.science.org/doi/10.1126/science.abj2949
Journal information: Science
Provided by European Molecular Biology Laboratory
A new study of gene expression in sponges reveals the complex diversity of their cells as well as some possibly ancient connections between the nervous, immune and digestive systems.

A new atlas of gene expression in the sponge Spongilla has revealed surprising levels of cellular diversity in these primitive animals.
Allexxandar / Dreamstime.com
Viviane Callier
November 4, 2021
When the first sponge genomes were sequenced in the early 2000s, researchers were surprised to find that sponges not only have roughly as many genes as humans and other complex creatures but also have many of the same genes. Sponges are among the earliest branching lineages on the evolutionary tree of animal life; their simple bodies don’t even have a pattern of symmetry or a set number of parts. The presence of those genes implied that the genetic information for functions like muscle contraction and the differentiation of neurons was much more ancient than muscles or nervous systems themselves.
But what were those genes doing in an animal without neurons or muscles? Researchers could only make educated guesses and investigate expression patterns on a painstaking gene-by-gene basis.
Today, however, a new study taking advantage of rapid advances in genomic technologies has illuminated where about 26,000 genes are expressed in the freshwater sponge Spongilla. This atlas of gene expression reveals the genetic configuration of cell types throughout the sponge’s body, including some cell types never described before. It offers important hints about how cell types evolved in the first place, and it may help to settle a long, thorny debate about whether neurons evolved just once or many times. The study appears in the latest issue of Science.
This ambitious paper “leapfrogs” over previous work, according to Scott Nichols, who studies sponge evolution at the University of Denver. “What is extraordinary about it is that really fascinating hypotheses have emerged from this data set,” he said. “But I would emphasize strongly that they need to be experimentally tested.”
The most exciting hypothesis concerns cells inside the sponge’s digestive chambers. The chambers are lined with distinctive cells called choanocytes, which have a collar of fingerlike protrusions (microvilli) and a flagellum. The choanocytes beat their flagella to regulate the flow of water through the digestive chamber, all the while feeding on small particles and debris the water carries. The digestive chambers also contain mobile “neuroid” cells that were described years ago, although their identity and function were mysterious.
Using high-throughput single-cell RNA sequencing technology, Detlev Arendt’s team at the European Molecular Biology Laboratory in Heidelberg discovered that choanocytes express genes that in neurons produce the postsynaptic “scaffolding” involved in receiving and responding to neurotransmitters. They also discovered that the mobile neuroid cells express a suite of genes that are typically active in the presynaptic bulb of a neuron. This led the researchers to hypothesize that the neuroid cells might be talking to the choanocytes, and that the neuroid cells’ job might be to patrol the microbial environment in the digestive chamber and regulate the choanocytes’ feeding behaviors accordingly.

Sponges have digestive chambers lined with cells called choanocytes. Waving their flagella to propel water through the chambers, the choanocytes digest small particles in the flow.
Caterina Longo, Bari University; source: doi.org/10.1371/journal.pone.0042392.g005
When Jacob Musser, the postdoctoral fellow in Arendt’s lab who led the project, stained the sponge to look at where exactly the pre- and postsynaptic genes were being expressed, he saw that the neuroid cells expressing presynaptic genes were indeed near the choanocytes expressing postsynaptic genes. In fact, the neuroid cells reached out pseudopod arms that seemed to touch the choanocytes.
“This was obviously really tantalizing,” Musser said. “But you can’t really tell what is going on.”
To get a more detailed picture of what the cells were doing, Musser and the team used focused ion beam electron microscopy at the X-ray synchrotron facility in Hamburg to get very high-resolution 3D images of the cells, which could distinguish cellular features as small as 15 nanometers, roughly the size of many folded proteins. They saw that projections from the neuroid cells enveloped the choanocytes’ microvilli collar and flagellum, and that the neuroid cells held vesicles like those in the presynaptic bulb of a neuron. They suspect the vesicles are probably releasing glutamate, a neurotransmitter.
But tempting as it is to imagine these sponges as having primitive synapses, the researchers never observed direct, stable contacts between the neuroid cells and choanocytes. The connections between the cells instead seem to be transient. Furthermore, the DNA of sponges lacks genes for some of the key ion channels needed to create an action potential — the sharp electrical signal that stimulates the release of neurotransmitters in neurons.
Nevertheless, because sponges have always been thought to lack anything even resembling a nervous system, the suggestion that they have cellular mechanisms with a deep evolutionary relationship to neurons “is an exciting path forward to connect sponge biology to neural cell biology, to understand where neuronal signaling came from at all in animals,” Nichols said.

A colorized micrograph of the cells in a sponge digestive chamber (left) reveals the interaction of a neuroid cell (magenta) with a choanocyte (green). In a magnified detail (right), the transient contact between the two cells could be suggestive of the synaptic contact between neurons.
Quanta Magazine; source: Jacob Musser, Giulia Mizzon, Constantin Pape, Nicole Schieber / EMBL
The origin of neurons and nervous systems — and in particular, the question of whether neurons arose once or multiple times — is one of the most contentious topics in the field of evolutionary developmental biology, according to Maria Antonietta Tosches, who studies the evolution of cell types in vertebrates at Columbia University and previously trained in Arendt’s lab. The findings from this new study seem to bear on that mystery because the researchers found presynaptic gene sets expressed in neuroid cells and postsynaptic genes expressed in choanocytes. (Both sets of genes were active in other cell types as well.) That fact suggests that the genetic modules responsible for both the sending and receiving ends of cell-cell communication systems were deployed in various types of ancestral animal cells. Neurons could therefore have evolved repeatedly and independently through different applications of these gene modules, Tosches said.
In fact, many multifunctional cells in sponges express modules of genes usually associated with specialized cells in more complex animals like vertebrates. For example, sponge neuroid cells not only express some of the presynaptic machinery of neurons, but also express immune genes. (It’s possible that if neuroid cells monitor the microbial content of the digestive chambers for sponges, these immune genes assist in that role.) Sponges also have cells called pinacocytes that contract in unison like muscle cells to squeeze the animal and expunge waste or unwanted debris; pinacocytes have some sensory machinery that responds to nitric oxide, a vasodilator.
“Nitric oxide is what relaxes our smooth muscle in our blood vessels, so when our blood vessels expand, that’s nitric oxide causing that relaxation,” Musser said. “And we’ve actually shown through experiments in the paper that nitric oxide is also regulating contractions in this sponge.” Like glutamate, nitric oxide might have been part of an early signaling mechanism to coordinate primitive behaviors in the sponge, he suggests.

EVOLUTION
Scientists Debate the Origin of Cell Types in the First Animals
JULY 17, 2019
“Our data are very consistent with this notion that a large number of important functional pieces of machinery existed early in animal evolution,” Musser said. “And a lot of early animal evolution was about starting to subdivide this out to different cells. But likely these very first cell types were very multifunctional, and they had to do multiple things.” The earliest animal cells, like their close relatives the protozoans, probably had to be cellular Swiss Army knives. As multicellular animals evolved, their cells may have taken on different roles, a division of labor that may have led to more specialized cell types. But different lineages of animals may have divvied things up differently and to different degrees.
If the mixing and matching of genetic modules was a crucial theme of early animal evolution, then comparing the arrangement and expression of those modules in different species could tell us about their history — and about possible limitations on how haphazardly they can be shuffled. One researcher looking for those answers is Arnau Sebé-Pedrós, who studies cell type evolution at the Center for Genomic Regulation in Barcelona and who published the first atlases of cell types in sponges, placozoans and comb jellies in 2018.
Sebé-Pedrós thinks that the spatial configuration of the genes along the chromosomes could be revelatory because genes located together can share regulatory machinery. “I’m absolutely shocked by the degree of conservation of the gene orders in animal genomes,” he said. He suspects that the need to co-regulate sets of functionally related genes keeps them in the same chromosomal neighborhood.
Scientists are still in the early days of learning how cell types evolve and relate to one another. But as important as it is to clarify the muddy origins of animal evolution, sponge cell atlases are also making a major contribution by revealing the possibilities in animal cell biology. “It is not just important for us to understand the very origin of animals,” Sebé-Pedrós said, “but also to understand things that may be radically different from anything else that we know about other animals.”
No comments:
Post a Comment