DW
7/10/24
Victor Ambros and Gary Ruvkun have been awarded the Nobel Prize in physiology or medicine for their research into microRNA.
Victor Ambros and Gary Ruvkun have been awarded the Nobel Prize in physiology or medicine for their research into microRNA.
Victor Ambros and Gary Ruvkun were announced as the joint recipients of the 2024 Nobel Prize in medicine or physiology on Monday
Image: Steffen Trumpf/dpa/picture alliance
American scientists Victor Ambros and Gary Ruvkun have been jointly awarded the Nobel Prize in medicine or physiology for their shared discovery of microRNA and the role it plays in post-transcriptional gene regulation.
At the announcement by the Nobel Assembly at the Karolinska Institutet in Sweden on Monday morning, Nobel Committee vice-chair Professor Olle Kämpe described the discovery of microRNA as "a tiny molecule that has opened a new field in gene regulation."
Though the pair worked in separate labs, their joint research focus led to them combining their resources to expand knowledge of microRNA and its role.
"The seminal discovery of microRNA has introduced a new and unexpected mechanism of gene regulation," said Kämpe.
"MicroRNAs are important for our understanding of embryological development, normal cell physiology and diseases such as cancer. As an example, tumors often perturb microRNA networks to grow."
American scientists Victor Ambros and Gary Ruvkun have been jointly awarded the Nobel Prize in medicine or physiology for their shared discovery of microRNA and the role it plays in post-transcriptional gene regulation.
At the announcement by the Nobel Assembly at the Karolinska Institutet in Sweden on Monday morning, Nobel Committee vice-chair Professor Olle Kämpe described the discovery of microRNA as "a tiny molecule that has opened a new field in gene regulation."
Though the pair worked in separate labs, their joint research focus led to them combining their resources to expand knowledge of microRNA and its role.
"The seminal discovery of microRNA has introduced a new and unexpected mechanism of gene regulation," said Kämpe.
"MicroRNAs are important for our understanding of embryological development, normal cell physiology and diseases such as cancer. As an example, tumors often perturb microRNA networks to grow."
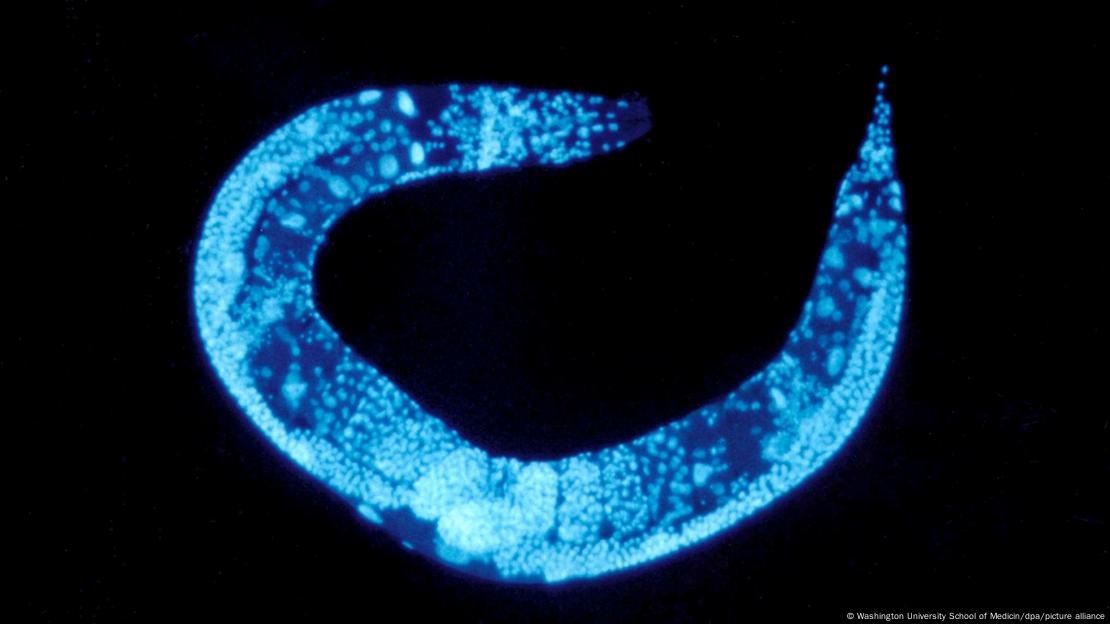
Mutations in the roundworm species Caenorhabditis elegans were the first signs of microRNA in living organisms
.Image: Washington University School of Medicin/dpa/picture alliance
Nobel Prize microRNA discovery started with a tiny roundworm
This Nobel Prize is all about foundational genetics.
At the heart of what makes a living organism function is the ability of double-stranded DNA to be translated by single-stranded RNA molecules. These "messenger" RNA (mRNA) create an "information molecule" from DNA and move into a cell’s protein factory — a ribosome — where amino acids align to this template and then fold into specialized proteins.
These proteins are the building blocks of all living organisms. But mutations or variations to genes can cause changes in function — often benign, but potentially disease-causing.
This general pathway to organism metabolism has been understood for a long time, but as Kämpe posed, "What determines that only the right genes are transcribed into messenger RNA and then translated into the right, tissue-specific proteins at the right time?"
The answer starts with one specific organism, the roundworm species Caenorhabditis elegans. Despite its size, the roundworm has 20,000 genes that code for proteins — about the same number as a human, making it an ideal lab ‘model’ for physiological research.
Different mutations to C. elegans genes were found to cause growth changes. One triggered excessive growth via a repeating developmental pathway. Another restricted growth due to a different gene variation.
Ambros found the enlarging "lin-4" variant in 1993, with Ruvkun isolating the "lin-14" mutation present in the miniature worms a year later. What wasn’t clear was how these variations interacted and influenced cell regulation. The pair joined forces to find the answer.
A micro discovery leads to big implications for science
Ambros and Ruvkun found their respective mutations interacted — specifically, that a sequence of code on the lin-4 gene corresponded to part of a lin-14 sequence.
This was the critical moment when microRNA was determined to exist, as a distinct form of RNA.
"At this point they had discovered a novel and unexpected mechanism of gene regulation — microRNA," said Kämpe. "For a long time, however, microRNA was believed to be an oddity peculiar to C. elegans."
It required more evidence to confirm their findings.
It came in 2000, when Ruvkun found another gene — "let-7" — which was found not just in roundworm, but in humans and most animals.
Many microRNAs, it turns out, are highly conserved across animals, plants and fungi, meaning that they are largely unchanged from species-to-species and across hundreds of millions of years of biological evolution.
More than 1,000 microRNA genes have been found in humans.
"Every microRNA regulates several genes," said Kämpe. "And each mRNA is regulated by many distinct microRNAs, creating a robust system for gene regulation."
When did RNA enter the public spotlight?
RNA was thrust into the public consciousness with the rise of RNA-based vaccine technology at the height of the COVID-19 pandemic.
These vaccine products could be developed relatively quickly by creating imitation proteins based on small sections of genetic code from the SARS-CoV-2 virus.
When used in a vaccine, these proteins provide a non-disease-causing target for the human immune system to find and create antibodies ready for the real virus.
Katalin Kariko and Drew Weissman were awarded last year’s prize for their work developing mRNA vaccine technology.
However while last year’s prize was very much in recognition of work that had led to direct medical applications, this year’s is more research focused.
"This year’s prize is definitely a physiology prize," said Professor Gunilla Karlsson Hederstam, chair of the Nobel Committee for Physiology or Medicine. "Last year, of course, [was] much a more applied discovery that was translated into vaccine development, so two quite different prizes.
"Although there are no very clear applications available yet, understanding them, knowing that they exist, understanding their regulatory networks is always the first step."

Joint laureate in the 2024 Nobel Prize for Physiology, Victor Ambros laughs with colleagues.
Image: Steven Senne/AP/picture alliance
Why type of products are being developed which utilize microRNA technology?
So while this year’s prize is very much focused on discovery rather than application, the realization of the Ambros-Ruvkun research may not be far away. There are currently several vaccine-type products in clinical trial stage for cancer, cardiovascular and other diseases that use microRNA technology.
The challenge is hitting the right target. Take a cancer cell. There may be a specific gene that a vaccine needs to address, but microRNAs regulate many different genes. The risk is that a product may act more like a bulldozer than a scalpel.
"But there might be ways around that," said Kämpe, "Tumors quite often perturb the microRNA networks and they can do that by deleting the genes or mutating the genes that process the microRNA.
"In [this] case there are promising first tests to see if you can modulate the RNA-binding proteins, but to deliver microRNAs to cells and think you get one effect, I think will be very difficult."
Two more Nobel science prizes will be awarded this week, with the physics laureate to be revealed on Tuesday, and chemistry prize on Wednesday.

Joint laureate in the 2024 Nobel Prize for Physiology, Gary Ruvkun.
Image: Steven Senne/AP/picture alliance
What is the history of the Nobel Prize in Physiology or Medicine?
This year's Prize, set at 11 million Swedish kronor (about $1.06 million USD), is yet another recognition of genetic discovery.
Arthur Kornberg and Severo Ochoa were recognized in 1959 for identifying the synthesis mechanisms of DNA and RNA, while the famed trio Crick, Watson and Wilkins were awarded the prize in 1952 for unravelling the DNA Double Helix.
Fire and Mello (2006), and Karikó and Weissman (2023) have also had their work on RNA recognized.
Famed Austrian neurologist and founder of psychoanalysis Sigmund Freud (1856-1939) was nominated for his work in Physiology and Medicine but was never named as a recipient.
At 31, Canadian surgeon and pharmacologist Frederick G. Banting is the youngest recipient of the Prize in Physiology or Medicine. He was recognized in 1923 for his discovery of insulin.
The American pathologist Francis Peyton Rous is the oldest, receiving his award in 1966 aged 87 for his discovery of tumor-inducing viruses.
The prize has been declined once. In 1939, Gerhard Domagk was prevented by Germany's Nazi Government from receiving his award for his discovery of an antibiotic against Streptococcus infections. He was later able to receive his diploma and medal in 1947.
Edited by Wesley Dockery
What is microRNA? Nobel-winning discovery explained
Agence France-Presse
October 7, 2024

Victor Ambros and Gary Ruvkun won the Nobel for medicine for their discovery of microRNA © Jonathan NACKSTRAND / AFPn/liVictor Ambros and Gary Ruvkun won the Nobel for medicine for their discovery of microRNA © Jonathan NACKSTRAND / AFP
The Nobel Prize in Medicine was awarded on Monday to two US scientists for discovering microRNA, a previously unknown type of genetic switch which is hoped can pave the way for new medical breakthroughs.
But while several treatments and tests are under development using microRNAs against cancer, heart disease, viruses and other illnesses, none have actually yet reached patients.
And the world paid little attention when the new Nobel laureates Victor Ambros and Gary Ruvkun revealed their discovery decades ago, thinking it was just "something weird about worms", Cambridge University geneticist Eric Miska told AFP.
Here is an explainer about how exactly these tiny genetic switches work inside our bodies.
What is microRNA?
Each cell in the human body has the same set of instructions, called DNA. Some turn into brain cells, while others become muscles.
So how do the cells know what to become? The relevant part of the DNA's instructions is pointed to via a process called gene regulation.
Ribonucleic acid (RNA) normally serves as a messenger. It delivers the instructions from the DNA to proteins, which are the building blocks of life that turn cells into brains -- or muscles.
Miska gave the example of the messenger RNA vaccines rolled out against Covid-19 during the pandemic, which insert a message with new instructions to build proteins that block viruses.

What did the Nobel winners do?
Back in the 1980s, Ambros and Ruvkun had been working separately on how genes interact in one-millimetre-long roundworms called C.elegans.
When they compared their work, it led to the discovery of microRNA. Ambros revealed the finding in a 1993 paper.
"Nobody really paid much attention," Miska said, explaining that most scientists at the time thought it only applied to worms.
Then in 2000, Ruvkun published research showing that microRNA is present right across the animal kingdom, including in humans and even some viruses.
"This was not just something weird that worms do, but in fact all animals and plants are totally dependent for development and normal function on them," Miska said.
More than a thousand genes that respond to microRNAs are now believed to be in the human body.
How could this help us?
There are numerous new treatments and tests using microRNA that are undergoing trials but none have been made widely available.
"Though there are no very clear applications available yet in microRNAs, understanding them, knowing that they exist, understanding their counter-regulatory networks, is always the first step," the Karolinska Institute's Gunilla Karlsson Hedestam told journalists in Stockholm.
MicroRNAs are particularly promising for fighting cancer because some of these switches "act as a tumour suppressor, so they put a brake on cells dividing inappropriately," Miska said.
Others, meanwhile, induce "cells to divide, which can lead to cancer", he added.
Because many viruses use microRNAs, several antiviral drugs are at varying stages of development, including for hepatitis C.
One complicating factor has been that microRNAs can be unstable.
But scientists also hope they can be used as a test called a "biomarker", which could reveal what type of cancer a patient could be suffering from, for example.
What next?
It also appears probable that microRNAs could be involved in the evolution of our species, Miska said.
While human brains are difficult to study, Miska hoped future research will discover more.
© 2024 AFP
Agence France-Presse
October 7, 2024

Victor Ambros and Gary Ruvkun won the Nobel for medicine for their discovery of microRNA © Jonathan NACKSTRAND / AFPn/liVictor Ambros and Gary Ruvkun won the Nobel for medicine for their discovery of microRNA © Jonathan NACKSTRAND / AFP
The Nobel Prize in Medicine was awarded on Monday to two US scientists for discovering microRNA, a previously unknown type of genetic switch which is hoped can pave the way for new medical breakthroughs.
But while several treatments and tests are under development using microRNAs against cancer, heart disease, viruses and other illnesses, none have actually yet reached patients.
And the world paid little attention when the new Nobel laureates Victor Ambros and Gary Ruvkun revealed their discovery decades ago, thinking it was just "something weird about worms", Cambridge University geneticist Eric Miska told AFP.
Here is an explainer about how exactly these tiny genetic switches work inside our bodies.
What is microRNA?
Each cell in the human body has the same set of instructions, called DNA. Some turn into brain cells, while others become muscles.
So how do the cells know what to become? The relevant part of the DNA's instructions is pointed to via a process called gene regulation.
Ribonucleic acid (RNA) normally serves as a messenger. It delivers the instructions from the DNA to proteins, which are the building blocks of life that turn cells into brains -- or muscles.
Miska gave the example of the messenger RNA vaccines rolled out against Covid-19 during the pandemic, which insert a message with new instructions to build proteins that block viruses.

Nobel prize for medicine 2024 © Valentina BRESCHI, Sylvie HUSSON, Lise KIENNEMANN, Thierno TOURE / AFP
But the two new Nobel winners Ambros and Ruvkun discovered a whole new type of gene regulator that had previously been overlooked by science.
Rather than being the messenger which relays information, microRNA instead acts as a switch to turn other genes off and on.
"This was a whole new level of control that we had totally missed," said Miska, who has worked on microRNA for two decades, including with the new Nobel laureates.
"The discovery of microRNAs brought an additional level of complexity by revealing that regions that were thought to be non-coding play a role in gene regulation," French researcher Benoit Ballester told AFP.
But the two new Nobel winners Ambros and Ruvkun discovered a whole new type of gene regulator that had previously been overlooked by science.
Rather than being the messenger which relays information, microRNA instead acts as a switch to turn other genes off and on.
"This was a whole new level of control that we had totally missed," said Miska, who has worked on microRNA for two decades, including with the new Nobel laureates.
"The discovery of microRNAs brought an additional level of complexity by revealing that regions that were thought to be non-coding play a role in gene regulation," French researcher Benoit Ballester told AFP.
What did the Nobel winners do?
Back in the 1980s, Ambros and Ruvkun had been working separately on how genes interact in one-millimetre-long roundworms called C.elegans.
When they compared their work, it led to the discovery of microRNA. Ambros revealed the finding in a 1993 paper.
"Nobody really paid much attention," Miska said, explaining that most scientists at the time thought it only applied to worms.
Then in 2000, Ruvkun published research showing that microRNA is present right across the animal kingdom, including in humans and even some viruses.
"This was not just something weird that worms do, but in fact all animals and plants are totally dependent for development and normal function on them," Miska said.
More than a thousand genes that respond to microRNAs are now believed to be in the human body.
How could this help us?
There are numerous new treatments and tests using microRNA that are undergoing trials but none have been made widely available.
"Though there are no very clear applications available yet in microRNAs, understanding them, knowing that they exist, understanding their counter-regulatory networks, is always the first step," the Karolinska Institute's Gunilla Karlsson Hedestam told journalists in Stockholm.
MicroRNAs are particularly promising for fighting cancer because some of these switches "act as a tumour suppressor, so they put a brake on cells dividing inappropriately," Miska said.
Others, meanwhile, induce "cells to divide, which can lead to cancer", he added.
Because many viruses use microRNAs, several antiviral drugs are at varying stages of development, including for hepatitis C.
One complicating factor has been that microRNAs can be unstable.
But scientists also hope they can be used as a test called a "biomarker", which could reveal what type of cancer a patient could be suffering from, for example.
What next?
It also appears probable that microRNAs could be involved in the evolution of our species, Miska said.
While human brains are difficult to study, Miska hoped future research will discover more.
© 2024 AFP

No comments:
Post a Comment