Using waste carbon dioxide to separate metals from ores
A combined team of researchers from the University of Lyon and the University of Turin has developed a way to use waste CO2 to separate metals used in products. In their paper published in the journal Nature Chemistry, the group describes their process and why they believe it can be used as a global warming mitigation tool.
Scientists have promoted the idea of using carbon capture and storage (CCS) as a way to reduce the amount of CO2 emitted into the atmosphere. CCS involves capturing the exhaust from a car or a factory, removing the CO2 and then storing it until scientists develop a use for it.
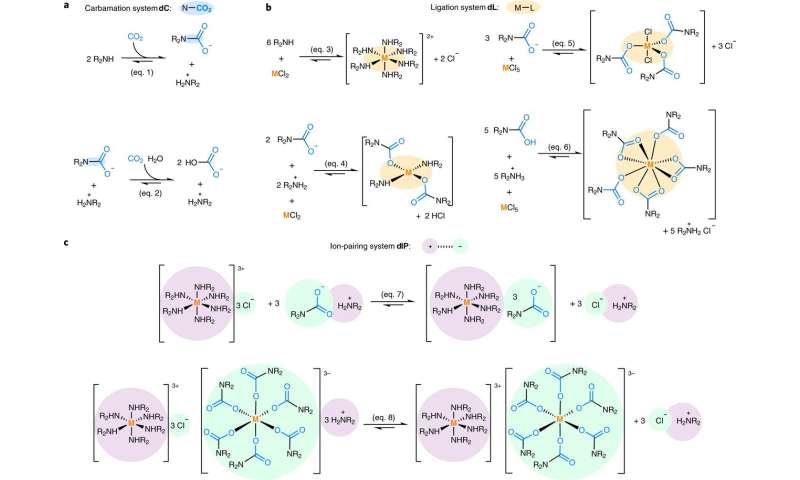
Unfortunately, CCS has proven to be too expensive for commercial use. In this new effort, the researchers developed a way to use waste CO2 to create ligands for separating metals from ores. The recovered metals can then be sold for use in making products such as smartphone components. Their idea is to recoup the cost of capturing CO2 (or make it profitable) so that businesses will find it more economically viable. The researchers claim their approach is the first to use two waste streams as part of a process that yields multiple purified compounds in a single pot.
In their process, the CO2 serves as a type of bonding agent—it takes advantage of the attraction of ligands for metals using temperature and pressure. The team injected 2,2'-Iminodi(ethylamine) solution into a mix of LaCl3 and NiCl2 to demonstrate how their approach works. They then bubbled CO2 from car exhaust through the mix. Doing so resulted in 2,2'-Iminodi(ethylamine) capturing carbon dioxide and producing ligands that bound with lanthanum.
After a few minutes, crystals containing lanthanum formed, and the nickel that was bound to unreacted diethylenetriamine remained in the solution. Both metals were then recovered using a centrifuge—testing showed both were 99 percent pure. A second test involved separating useful metals from an electrode taken from a nearly dead battery—it yielded cobalt, nickel and lanthanum. The researchers claim a secondary benefit of their approach is that it is a greener way to separate metals from ores than standard methods.
More information: Jean Septavaux et al. Simultaneous CO2 capture and metal purification from waste streams using triple-level dynamic combinatorial chemistry, Nature Chemistry (2020). DOI: 10.1038/s41557-019-0388-5
Journal information: Nature Chemistry
No comments:
Post a Comment