'Fool's gold' may be valuable after allby University of Minnesota
More information: Jeff Walter et al. Voltage-induced ferromagnetism in a diamagnet, Science Advances (2020). DOI: 10.1126/sciadv.abb7721
Journal information: Science Advances
Provided by University of Minnesota
Manipulating non-magnetic atoms in a chromium halide enables tuning of magnetic properties
by Boston College
More information: Accessing new magnetic regimes by tuning the ligand spin-orbit coupling in van der Waals magnets, Science Advances (2020). DOI: 10.1126/sciadv.abb9379
Journal information: Science Advances
Provided by Boston College

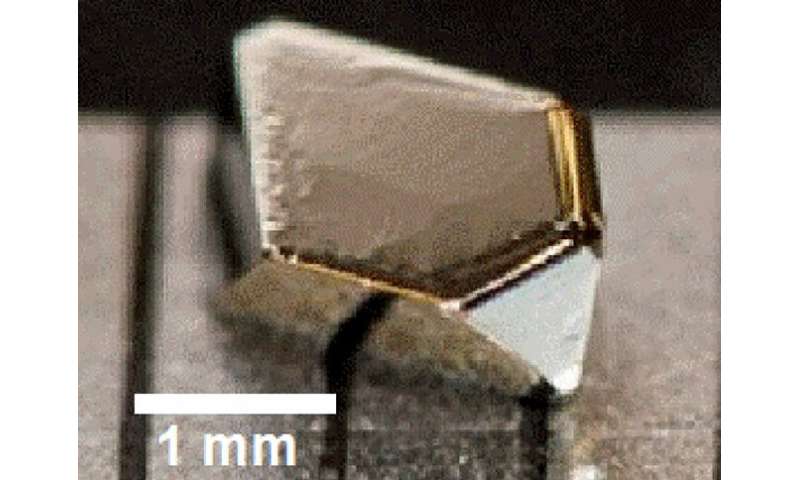
This image shows an example of a crystal of iron sulfide grown in the University of Minnesota lab to extremely high purity using a method called chemical vapor transport. Note the “goldish” sheen, which is characteristic of pyrite, or fool’s gold. Credit: University of Minnesota
In a breakthrough new study, scientists and engineers at the University of Minnesota have electrically transformed the abundant and low-cost non-magnetic material iron sulfide, also known as "fool's gold" or pyrite, into a magnetic material.
This is the first time scientists have ever electrically transformed an entirely non-magnetic material into a magnetic one, and it could be the first step in creating valuable new magnetic materials for more energy-efficient computer memory devices.
The research is published in Science Advances, a peer-reviewed scientific journal published by the American Association for the Advancement of Science (AAAS).
"Most people knowledgeable in magnetism would probably say it was impossible to electrically transform a non-magnetic material into a magnetic one. When we looked a little deeper, however, we saw a potential route, and made it happen," said Chris Leighton, the lead researcher on the study and a University of Minnesota Distinguished McKnight University Professor in the Department of Chemical Engineering and Materials Science.
Leighton and his colleagues, including Eray Aydil at New York University and Laura Gagliardi (chemistry) at the University of Minnesota, have been studying iron sulfide, or "fool's gold," for more than a decade for possible use in solar cells. Sulfur in particular is a highly abundant and low-cost byproduct of petroleum production. Unfortunately, scientists and engineers haven't found a way to make the material efficient enough to realize low-cost, earth-abundant solar cells.
"We really went back to the iron sulfide material to try to figure out the fundamental roadblocks to cheap, non-toxic solar cells," Leighton said. "Meanwhile, my group was also working in the emerging field of magnetoionics where we try to use electrical voltages to control magnetic properties of materials for potential applications in magnetic data storage devices. At some point we realized we should be combining these two research directions, and it paid off."
Leighton said their goal was to manipulate the magnetic properties of materials with a voltage alone, with very little electrical current, which is important to make magnetic devices more energy-efficient. Progress to date had included turning on and off ferromagnetism, the most technologically important form of magnetism, in other types of magnetic materials. Iron sulfide, however, offered the prospect of potentially electrically inducing ferromagnetism in an entirely non-magnetic material.
In the study, the researchers used a technique called electrolyte gating. They took the non-magnetic iron sulfide material and put it in a device in contact with an ionic solution, or electrolyte, comparable to Gatorade. They then applied as little as 1 volt (less voltage than a household battery), moved positively charged molecules to the interface between the electrolyte and the iron sulfide, and induced magnetism. Importantly, they were able to turn off the voltage and return the material to its non-magnetic state, meaning that they can reversibly switch the magnetism on and off.
"We were pretty surprised it worked," Leighton said. "By applying the voltage, we essentially pour electrons into the material. It turns out that if you get high enough concentrations of electrons, the material wants to spontaneously become ferromagnetic, which we were able to understand with theory. This has lots of potential. Having done it with iron sulfide, we guess we can do it with other materials as well."
Leighton said they would never have imagined trying this approach if it wasn't for his team's research studying iron sulfide for solar cells and the work on magnetoionics.
"It was the perfect convergence of two areas of research," he said.
Leighton said the next step is to continue research to replicate the process at higher temperatures, which the team's preliminary data suggest should certainly be possible. They also hope to try the process with other materials and to demonstrate potential for real devices.
In a breakthrough new study, scientists and engineers at the University of Minnesota have electrically transformed the abundant and low-cost non-magnetic material iron sulfide, also known as "fool's gold" or pyrite, into a magnetic material.
This is the first time scientists have ever electrically transformed an entirely non-magnetic material into a magnetic one, and it could be the first step in creating valuable new magnetic materials for more energy-efficient computer memory devices.
The research is published in Science Advances, a peer-reviewed scientific journal published by the American Association for the Advancement of Science (AAAS).
"Most people knowledgeable in magnetism would probably say it was impossible to electrically transform a non-magnetic material into a magnetic one. When we looked a little deeper, however, we saw a potential route, and made it happen," said Chris Leighton, the lead researcher on the study and a University of Minnesota Distinguished McKnight University Professor in the Department of Chemical Engineering and Materials Science.
Leighton and his colleagues, including Eray Aydil at New York University and Laura Gagliardi (chemistry) at the University of Minnesota, have been studying iron sulfide, or "fool's gold," for more than a decade for possible use in solar cells. Sulfur in particular is a highly abundant and low-cost byproduct of petroleum production. Unfortunately, scientists and engineers haven't found a way to make the material efficient enough to realize low-cost, earth-abundant solar cells.
"We really went back to the iron sulfide material to try to figure out the fundamental roadblocks to cheap, non-toxic solar cells," Leighton said. "Meanwhile, my group was also working in the emerging field of magnetoionics where we try to use electrical voltages to control magnetic properties of materials for potential applications in magnetic data storage devices. At some point we realized we should be combining these two research directions, and it paid off."
Leighton said their goal was to manipulate the magnetic properties of materials with a voltage alone, with very little electrical current, which is important to make magnetic devices more energy-efficient. Progress to date had included turning on and off ferromagnetism, the most technologically important form of magnetism, in other types of magnetic materials. Iron sulfide, however, offered the prospect of potentially electrically inducing ferromagnetism in an entirely non-magnetic material.
In the study, the researchers used a technique called electrolyte gating. They took the non-magnetic iron sulfide material and put it in a device in contact with an ionic solution, or electrolyte, comparable to Gatorade. They then applied as little as 1 volt (less voltage than a household battery), moved positively charged molecules to the interface between the electrolyte and the iron sulfide, and induced magnetism. Importantly, they were able to turn off the voltage and return the material to its non-magnetic state, meaning that they can reversibly switch the magnetism on and off.
"We were pretty surprised it worked," Leighton said. "By applying the voltage, we essentially pour electrons into the material. It turns out that if you get high enough concentrations of electrons, the material wants to spontaneously become ferromagnetic, which we were able to understand with theory. This has lots of potential. Having done it with iron sulfide, we guess we can do it with other materials as well."
Leighton said they would never have imagined trying this approach if it wasn't for his team's research studying iron sulfide for solar cells and the work on magnetoionics.
"It was the perfect convergence of two areas of research," he said.
Leighton said the next step is to continue research to replicate the process at higher temperatures, which the team's preliminary data suggest should certainly be possible. They also hope to try the process with other materials and to demonstrate potential for real devices.
More information: Jeff Walter et al. Voltage-induced ferromagnetism in a diamagnet, Science Advances (2020). DOI: 10.1126/sciadv.abb7721
Journal information: Science Advances
Provided by University of Minnesota
Manipulating non-magnetic atoms in a chromium halide enables tuning of magnetic properties
by Boston College

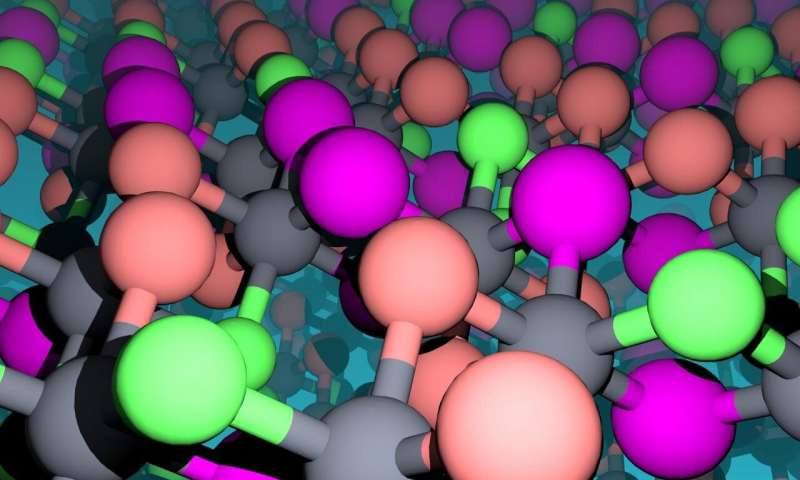
The atomic landscape of chromium halides are illustrated. The magnetic chromium atoms appear as gray spheres and the non-magnetic ligand atoms as green (chlorine), orange (bromine), and magenta (iodine) spheres. Credit: Fazel Tafti
The magnetic properties of a chromium halide can be tuned by manipulating the non-magnetic atoms in the material, a team, led by Boston College researchers, reports in the most recent edition of Science Advances.
The seemingly counter-intuitive method is based on a mechanism known as an indirect exchange interaction, according to Boston College Assistant Professor of Physics Fazel Tafti, a lead author of the report.
An indirect interaction is mediated between two magnetic atoms via a non-magnetic atom known as the ligand. The Tafti Lab findings show that by changing the composition of these ligand atoms, all the magnetic properties can be easily tuned.
"We addressed a fundamental question: is it possible to control the magnetic properties of a material by changing the non-magnetic elements?" said Tafti. "This idea and the methodology we report on are unprecedented. Our findings demonstrate a new approach to create synthetic layered magnets with unprecedented level of control over their magnetic properties."
Magnetic materials are the backbone of most current technology, such as the magnetic memory in our mobile devices. It is common practice to tune the magnetic properties by modifying the magnetic atoms in a material. For example, one magnetic element, such as chromium, can be replaced with another one, such as iron.
The team studied ways to experimentally control the magnetic properties of inorganic magnetic materials, specifically, chromium halides. These materials are made of one Chromium atom and three halide atoms: Chlorine, Bromine, and Iodine.
The central finding illustrates a new method of controlling the magnetic interactions in layered materials by using a special interaction known as the ligand spin-orbit coupling. The spin-orbit coupling is a property of an atom to re-orient the direction of spins—the tiny magnets on the electrons—with the orbital movement of the electrons around the atoms.
This interaction controls the direction and magnitude of magnetism. Scientists have been familiar with the spin-orbit coupling of the magnetic atoms, but they did not know that the spin-orbit coupling of the non-magnetic atoms could also be utilized to re-orient the spins and tune the magnetic properties, according to Tafti.
The team was surprised that they could generate an entire phase diagram by modifying the non-magnetic atoms in a compound, said Tafti, who co-authored the report with fellow BC physicists Ying Ran and Kenneth Burch, post-doctoral researchers Joseph Tang and Mykola Abramchuk, graduate student Faranak Bahrami, and undergraduate students Thomas Tartaglia and Meaghan Doyle. Julia Chan and Gregory McCandless of the University of Texas, Dallas, and Jose Lado of Finland's Aalto University, were also part of the team.
"This finding puts forward a novel procedure to control magnetism in layered materials, opening up a pathway to create new synthetic magnets with exotic properties," Tafti said. "Moreover, we found strong signatures of a potentially exotic quantum state associated to magnetic frustration, an unexpected discovery that can lead to an exciting new research direction."
Tafti said the next step is to use these materials in innovative technologies such as magneto-optical devices or the new generation of magnetic memories.
Explore further Mixed halide chemistry can be used to control magnetism in ultrathin magnetic devices
The magnetic properties of a chromium halide can be tuned by manipulating the non-magnetic atoms in the material, a team, led by Boston College researchers, reports in the most recent edition of Science Advances.
The seemingly counter-intuitive method is based on a mechanism known as an indirect exchange interaction, according to Boston College Assistant Professor of Physics Fazel Tafti, a lead author of the report.
An indirect interaction is mediated between two magnetic atoms via a non-magnetic atom known as the ligand. The Tafti Lab findings show that by changing the composition of these ligand atoms, all the magnetic properties can be easily tuned.
"We addressed a fundamental question: is it possible to control the magnetic properties of a material by changing the non-magnetic elements?" said Tafti. "This idea and the methodology we report on are unprecedented. Our findings demonstrate a new approach to create synthetic layered magnets with unprecedented level of control over their magnetic properties."
Magnetic materials are the backbone of most current technology, such as the magnetic memory in our mobile devices. It is common practice to tune the magnetic properties by modifying the magnetic atoms in a material. For example, one magnetic element, such as chromium, can be replaced with another one, such as iron.
The team studied ways to experimentally control the magnetic properties of inorganic magnetic materials, specifically, chromium halides. These materials are made of one Chromium atom and three halide atoms: Chlorine, Bromine, and Iodine.
The central finding illustrates a new method of controlling the magnetic interactions in layered materials by using a special interaction known as the ligand spin-orbit coupling. The spin-orbit coupling is a property of an atom to re-orient the direction of spins—the tiny magnets on the electrons—with the orbital movement of the electrons around the atoms.
This interaction controls the direction and magnitude of magnetism. Scientists have been familiar with the spin-orbit coupling of the magnetic atoms, but they did not know that the spin-orbit coupling of the non-magnetic atoms could also be utilized to re-orient the spins and tune the magnetic properties, according to Tafti.
The team was surprised that they could generate an entire phase diagram by modifying the non-magnetic atoms in a compound, said Tafti, who co-authored the report with fellow BC physicists Ying Ran and Kenneth Burch, post-doctoral researchers Joseph Tang and Mykola Abramchuk, graduate student Faranak Bahrami, and undergraduate students Thomas Tartaglia and Meaghan Doyle. Julia Chan and Gregory McCandless of the University of Texas, Dallas, and Jose Lado of Finland's Aalto University, were also part of the team.
"This finding puts forward a novel procedure to control magnetism in layered materials, opening up a pathway to create new synthetic magnets with exotic properties," Tafti said. "Moreover, we found strong signatures of a potentially exotic quantum state associated to magnetic frustration, an unexpected discovery that can lead to an exciting new research direction."
Tafti said the next step is to use these materials in innovative technologies such as magneto-optical devices or the new generation of magnetic memories.
Explore further Mixed halide chemistry can be used to control magnetism in ultrathin magnetic devices
More information: Accessing new magnetic regimes by tuning the ligand spin-orbit coupling in van der Waals magnets, Science Advances (2020). DOI: 10.1126/sciadv.abb9379
Journal information: Science Advances
Provided by Boston College
No comments:
Post a Comment