Before geoengineering to mitigate climate change, researchers must consider some fundamental chemistry
It's a tempting thought: With climate change so difficult to manage and nations unwilling to take decisive action, what if we could mitigate its effects by setting up a kind of chemical umbrella—a layer of sulfuric acid in the upper atmosphere that could reflect the sun's radiation and cool the Earth?
According to a new study in the Journal of the American Chemical Society, a collaboration among Penn scientists and two groups in Spain, atmospheric conditions in the stratosphere pose a challenge to generating sulfuric acid, making its production less efficient than might have previously been expected. Thus more groundwork exploring the chemistry of how sulfuric acid and its building blocks will react in the upper atmosphere is required in order to confidently move forward with this climate geoengineering strategy, the researchers say.
"These fundamental insights highlight the importance of understanding the photochemistry involved in geoengineering," says Joseph S. Francisco, an atmospheric chemist in Penn's School of Arts & Sciences and a co-corresponding author on the study. "That's critically important and it's something that's been ignored."
Using sulfuric acid to blunt the sun's rays as a means of curbing climate change impacts is based on a natural phenomenon: When volcanoes erupt, the sulfur they emit creates localized—or sometimes even far-reaching—cooling clouds that filter the sun. But those clouds emerge in the troposphere, which ranges from the Earth's surface to about 10 kilometers up. Geoengineering using sulfuric acid would happen a good deal higher, in the stratosphere, from about 10 to 20 kilometers above the planet.
Conditions change as the altitude increases. Notably, the air becomes drier, and the energy of the sun's rays becomes stronger. In the new work, Francisco, his postdoc Tarek Trabelsi, and colleagues from Spain's Rocasolano Institute of Physical Chemistry and the University of València partnered to explore how these variables affected the chemical reactions involved in making sulfuric acid.
The major inputs are sulfur dioxide (SO2), which reacts with hydroxyl radicals (OH), a kind of atmospheric "detergent," to create HOSO2. HOSO2 reacts with oxygen to create sulfur trioxide (SO3), which then reacts with water vapor to create sulfuric acid. Aerosols formed from the sulfuric acid have the ability to reflect sunlight.
These reactions are well characterized; together, they are responsible for creating acid rain in the troposphere. But whether that chemistry would work in the stratosphere and achieve the same efficiency was unknown.
To find out, the team used quantum chemistry—an approach that considers the ground, transition, and excited states of atoms and molecules—to consider how HOSO2 and SO3 would behave in the stratosphere's conditions of high light and low humidity. Though geoengineering approaches factor in the ability of these two molecules to reflect sunlight, the researchers found that when HOSO2 is produced in the stratosphere, solar radiation causes the molecule to quickly photolyze, essentially breaking apart into its component parts, including sulfur dioxide, which is harmful to humans in high concentrations.
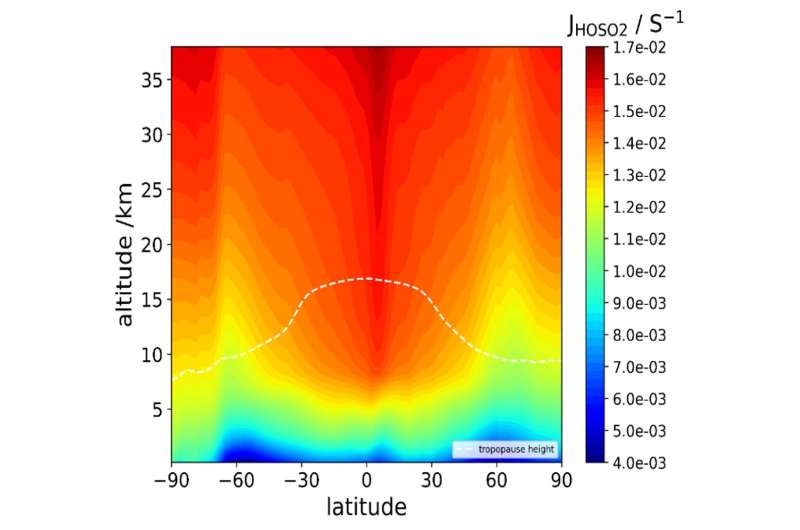
"One of the implications of this finding is, if you put sulfur dioxide up there, it's going to just be recycling around," Francisco says. "So it opens the door to whether we have a full understanding of atmospheric sulfur chemistry up in the stratosphere."
Declining HOSO2 would also blunt the efficiency of producing sulfuric acid, the researchers note, possibly lessening the effectiveness of a chemical sunshade.
In contrast, the researchers found that SO3 levels remained quite stable in stratospheric conditions. "We know it reacts with water, but we don't know a lot else about how it might react," says Francisco. "Will the atmosphere find a way to get rid of the SO3 or will it collect somewhere and start initiating new chemistry elsewhere?"
Indeed, the researchers note that it's crucial to understand what other reactions these molecules could be entering into in the stratosphere. "This work points to a cautionary note: If the SO3 chemistry is different, then how does it interact with the other chemistry that's currently going on in the stratosphere," he says. "We need to consider whether there are any kind of chemical concerns that we need to think about up front."
The findings also highlight the need for a Plan B if the atmospheric chemistry doesn't play out as expected. "It raises a fundamentally important question," Francisco says. "If we put the sulfur dioxide in, can we get it out of the stratosphere?"
Francisco's group is working on continuing to apply cutting-edge quantum methodologies to examine how photochemistry interacts with atmospheric models to generate a more complete understanding of various geoengineering scenarios.
"This is the first time that you're taking results from fundamental physics and chemistry and mapping them into climate models to look at the three-dimensional atmospheric impact," Francisco says.
And while some scientists are already proposing to trial a geoengineering approach using SO2, Francisco and his colleagues underscore that the outcomes depend on some aspects of sulfur chemistry that remain unknown.
"This brings to the forefront the need to make the community aware that there's more fundamental chemistry that we need before we start to understand the full chemical impact of this approach," Francisco says.Mechanism deciphered: How organic acids are formed in the atmosphere\
More information: Javier Carmona-García et al, Photochemistry of HOSO2 and SO3 and Implications for the Production of Sulfuric Acid, Journal of the American Chemical Society (2021). DOI: 10.1021/jacs.1c10153
Journal information: Journal of the American Chemical Society
Provided by University of Pennsylvania







