Diazotrophs are overlooked contributors to carbon and nitrogen export to the deep ocean
The ISME Journal (2022)
Abstract
Diazotrophs are widespread microorganisms that alleviate nitrogen limitation in 60% of our oceans, thereby regulating marine productivity. Yet, the group-specific contribution of diazotrophs to organic matter export has not been quantified, which so far has impeded an accurate assessment of their impact on the biological carbon pump. Here, we examine the fate of five groups of globally-distributed diazotrophs by using an original combination of mesopelagic particle sampling devices across the subtropical South Pacific Ocean. We demonstrate that cyanobacterial and non-cyanobacterial diazotrophs are exported down to 1000 m depth. Surprisingly, group-specific export turnover rates point to a more efficient export of small unicellular cyanobacterial diazotrophs (UCYN) relative to the larger and filamentous Trichodesmium. Phycoerythrin-containing UCYN-B and UCYN-C-like cells were recurrently found embedded in large (>50 µm) organic aggregates or organized into clusters of tens to hundreds of cells linked by an extracellular matrix, presumably facilitating their export. Beyond the South Pacific, our data are supported by analysis of the Tara Oceans metagenomes collected in other ocean basins, extending the scope of our results globally. We show that, when diazotrophs are found in the euphotic zone, they are also systematically present in mesopelagic waters, suggesting their transport to the deep ocean. We thus conclude that diazotrophs are a significant part of the carbon sequestered in the deep ocean and, therefore, they need to be accounted in regional and global estimates of export.
Introduction
Nitrogen (N) availability limits primary productivity throughout much of the surface low-latitude ocean [1]. In such nitrogen (N)-limited waters, microbial dinitrogen (N2) fixation by diazotrophic plankton provides the major source of new N to the surface ocean [2], maintaining ocean fertility and, on appropriate timescales, is equivalent to export production to the deep ocean [3]. However, the fate of this production remains obscure [4, 5]. Currently, there is no consensus as to whether diazotrophically fixed N is exported to the deep ocean or if it stimulates remineralization in surface waters. An increasing number of studies have shown that diazotroph-derived N is quickly translocated to non-diazotrophic plankton such as diatoms [6, 7], which eventually contributes to secondary export of organic matter out of the photic zone. Yet, except for Diatom-Diazotroph Associations (DDAs) [8], the direct gravitational settling of diazotrophs themselves to the deep ocean has rarely been quantified.
Diazotrophs may associate with sinking particles and contribute to direct export by different mechanisms. The most direct ones include gravitational settling of individual cells/filaments or aggregates. According to the Stokes’ law, particle sinking velocity scales with the square of particle size. Therefore, large particles should sink faster and are more likely to reach the deep ocean before being remineralized by bacteria [9]. However, past studies have revealed the importance of small (<2 µm) phytoplankton (including the non-diazotrophic Synechococcus and Prochlorococcus) in carbon export, especially in oligotrophic ocean regions [10, 11]. Aggregation is one of the crucial steps for the transport of these small plankton, which could export particulate organic carbon (POC) in similar proportion to their production in surface waters [11]. Diazotrophs have diverse morphologies and their size spans several orders of magnitude. Some types such as the free-living unicellular diazotrophic cyanobacteria (UCYN from groups B and C) are small (2–8 μm in diameter), while others such as Trichodesmium sp. are filamentous and can form large-sized colonies (>100–1000 µm). In addition, some diazotrophs live in symbiosis with calcified (UCYN-A, ~1 μm) or silicified eukaryotes (Richelia sp., Calothrix sp., >20 µm, forming DDAs). These dense biominerals may provide ballast enhancing the downward export of these symbioses into the deep ocean. Therefore, the presence of different diazotrophs in surface waters may result in drastically different export fluxes. Yet, to date, no field observations have explored how efficiently diazotrophs are exported, and if some are exported more efficiently than others, which prevents robust predictions of how diazotrophs contribute to the biological carbon pump.
Thanks to their inherently ballasted character, DDAs are well known to contribute to particulate matter export [12] and are involved in seasonal peaks of POC export to the deep sea (4000 m) in the North Pacific subtropical gyre [8]. Trichodesmium, one of the major contributors to global N2 fixation [13], is thought to have a limited export capacity and to be preferentially remineralized in the surface layers due to the presence of gas vesicles providing them buoyancy [4, 14, 15]. Yet, some studies have reported its presence in sediment traps material in the Kuroshio Current [16], the tropical North [17] and South Pacific Oceans [18]. Intact filaments and colonies of Trichodesmium sp. have also been reported as deep as 3000–4000 m in the tropical Atlantic, Pacific and Indian Oceans [19, 20], but their contribution to organic matter export has yet to be quantified.
Theoretically, UCYN may not contribute significantly to POC export fluxes due to their small size. Yet, Berthelot et al. [21] reported that primary production supported by UCYN was twice as efficient in promoting POC export than production supported by DDAs. Recent studies confirmed the presence of UCYN-B in sinking particles in the meso- and bathypelagic ocean [18, 22], but the export of such small particles (1–8 µm) remains enigmatic. Finally, Farnelid et al. [23] observed nifH gene sequences in exported material (150 m) in the North Pacific subtropical gyre and found that all the diazotroph groups above as well as non-cyanobacterial diazotrophs were present in the samples.
Taken together, these studies suggest that all diazotroph groups, small or large, free-living or symbiotic, ballasted or not, have been detected below the photic layer, raising the question of their potential impact on the biological carbon pump. A detailed examination relating diazotroph types to the magnitude of downward biogenic elements fluxes is needed to refine our understanding of their role in the mechanisms controlling the export of organic matter in the ocean. This is a pressing question as diazotrophs have recently been identified as key drivers influencing the response of future marine net primary productivity to climate change [24].
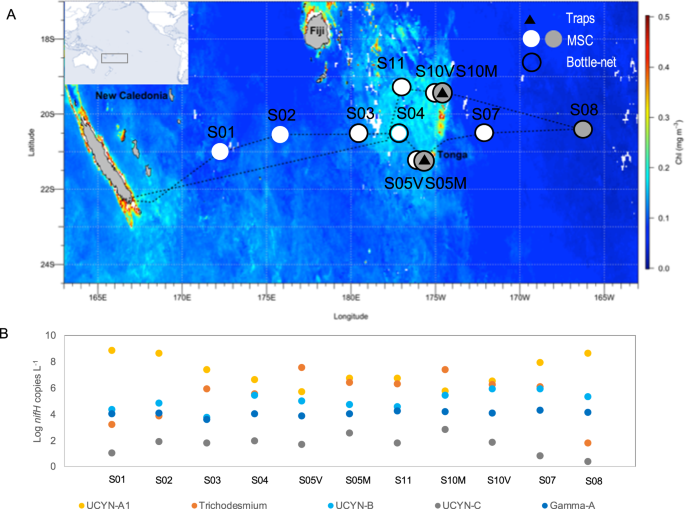
Here, we examine the group-specific fate of diazotrophs in the mesopelagic ocean. We used an innovative approach consisting of the combined deployment of surface-tethered drifting sediment traps, a Marine Snow Catcher (MSC), and a Bottle-net, in which we performed nifH gene sequencing and quantitative PCR on major diazotroph groups across the subtropical South Pacific Ocean (Fig. 1A) in parallel with export flux quantification. We show that all globally significant N2-fixing cyanobacteria and non-cyanobacterial diazotrophs are systematically present in sinking particles down to 1000 m. Small size UCYN (1–8 µm) are exported more efficiently than large filamentous diazotrophs under the form of large (>50 µm) aggregates linked by an extracellular matrix. Globally, our analysis of the Tara Oceans metagenomes confirms that diazotrophs are always detected in mesopelagic waters when present in surface waters, potentially revealing that the transport of diazotrophs to the deep ocean is an important pathway for diazotroph-derived export production, influencing the ability of our ocean to sequester carbon.
A Satellite-derived surface chlorophyll a concentrations during the GPpr14 cruise (1 November-6 December 2019) (MODIS Aqua, 4 km, 8-days composite, level 3 product). Black triangles correspond to stations where surface-tethered drifting sediment traps were deployed (170 m, 270 m, 1000 m). Grey dots correspond to stations where Marine Snow Catcher (MSC) casts were performed at three depths (see Methods), and white dots to MSC casts performed at one depth (200 m). Black circles correspond to stations where the bottlenet profiles were performed between 2000 m and 200 m. B Abundances (Log10 nifH gene copies L−1) of the five nifH phylotypes targeted over the transect (dots represent abundances averaged over the photic layer, ~0–100 m).
Materials and methods
Samples were collected during the GEOTRACES GPpr14 expedition (https://doi.org/10.17600/18000884) in the subtropical South Pacific Ocean (Fig. 1) in austral summer (Nov.-Dec. 2019).
READ ON/DOWNLOAD PDF