MICROVERSE
Attack and defence in the microverse
How small RNA molecules regulate viral infections of bacteria
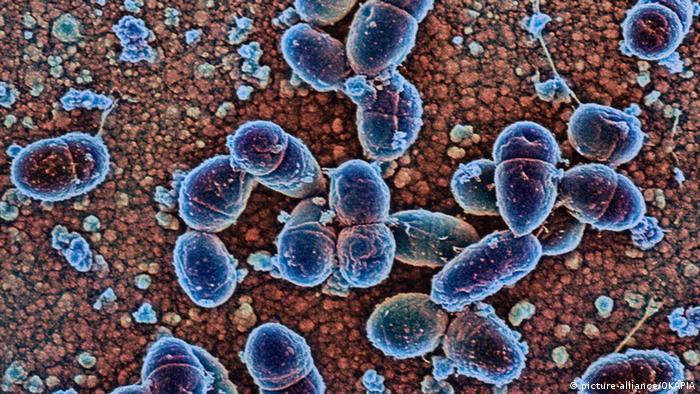
IMAGE:
VIEW OF A PETRI DISH WITH CHOLERA BACTERIA (VIBRIO CHOLERAE).
view moreCREDIT: JENS MEYER/UNIVERSITY OF JENA
Viruses need hosts. Whether it’s measles, the flu or coronavirus, viral pathogens cannot multiply or infect other organisms without the assistance of their hosts’ cellular infrastructure. However, humans are not the only ones affected by viruses: animals, plants and even microorganisms can all serve as hosts. Viruses that use bacteria as host cells are called bacteriophages (or simply “phages” for short) and are thought to be the most abundant biological entities of all. Just as the human immune system springs into action to resist a flu or coronavirus infection, bacteria do not simply allow phages to infiltrate their cellular machinery without a fight.
A research team at the University of Jena and its Cluster of Excellence “Balance of the Microverse” has examined in detail the complex interaction of attack and defence strategies when cholera-causing bacteria (Vibrio cholerae) are infected with a bacteriophage known as VP882—and discovered that tiny RNA molecules play a decisive role. The researchers’ findings have been published in the latest issue of a prestigious journal, Cell Host & Microbe.
From harmless housemate to cunning kidnapper
There are two ways in which phages can multiply after infecting bacteria: either as invisible passengers, hidden in the bacteria’s genetic material, or as cunning kidnappers, multiplying in vast numbers in bacterial cells without regard for potential losses and, ultimately, destroying the cells. Which method a phage adopts depends on whether sufficient numbers of other host cells are available in the immediate environment to provide shelter.
But how do phages determine this? “They rely on a chemical counting mechanism that bacteria use to identify other members of their species,” explains Prof. Dr Kai Papenfort of the University of Jena, who headed up the project. Known as “quorum sensing”, this method uses signal molecules that are produced by bacteria and released into their surroundings. At the same time, the bacteria monitor the concentration of these molecules using specific receptors, thereby gaining information about the size of their current population. “The phages’ trick essentially involves ‘listening in’ to this chemical communication between bacteria,” says Papenfort.
In their experiments, the Jena researchers examined what happens to the phages and bacteria once the bacteria emit their quorum sensing signals. “We have observed that 99% of bacteria are destroyed within 60 minutes, in which time the phages take control,” reports Dr Marcel Sprenger, the lead author of the article. The team discovered that this switchover is controlled by tiny RNA molecules, one of which is called “VpdS” (VP882 phage-derived sRNA). “As soon as the phages receive the chemical signal from the bacteria, this RNA is produced in high quantities,” says Sprenger.
How bacteria fight back against viruses
In order to find out precisely which genes are regulated by VpdS, the team adopted a comprehensive, technological approach and infected bacteria cultures with both VP882 phages and genetically modified phages unable to produce VpdS. Applying a method known as “RNA interaction by ligation and sequencing”, the researchers were able to identify the interactions between all RNA molecules in the bacteria cultures at different times. “This not only gave us insights into which genes are active, it also showed how they interact,” says Papenfort.
This method enabled the researchers to examine the genes of the phages as well as those of the host bacteria. As a result, the researchers gained extensive insights into the changes that occurred both during and after quorum sensing. “We were able to demonstrate that VpdS regulates phage genes as well as genes of the host, which effectively explains the destruction of bacterial cells,” says Papenfort.
However, the researchers have been able to deduce further relationships from the data they collected. For example, bacteria also have genes that, when activated by a chemical signal, fight back against the phages’ propagation and thereby counteract their own destruction. According to Papenfort, this aspect is particularly interesting. “We can see these as the precursors to the immune systems in higher organisms. Bacteria have many genes that protect them against viruses.” Given that these genes are also present in higher organisms, the researchers surmise that RNA molecules could also play an important role in their regulation.
Original publication:
Sprenger M. et al.: Small RNAs direct attack and defence mechanisms in a quorum sensing phage and its host. Cell. Host & Microbe (2024), https://doi.org/10.1016/j.chom.2024.03.010
Contact:
Prof. Dr Kai Papenfort
Institute of Microbiology at Friedrich Schiller University Jena
Winzerlaer Straße 2, 07745 Jena, Germany
Phone: +49 (0)3641 9 49311
Email: kai.papenfort@uni-jena.de
JOURNAL
Cell Host & Microbe
METHOD OF RESEARCH
Experimental study
SUBJECT OF RESEARCH
Cells
ARTICLE TITLE
Small RNAs direct attack and defence mechanisms in a quorum sensing phage and its host.
ARTICLE PUBLICATION DATE
4-Apr-2024
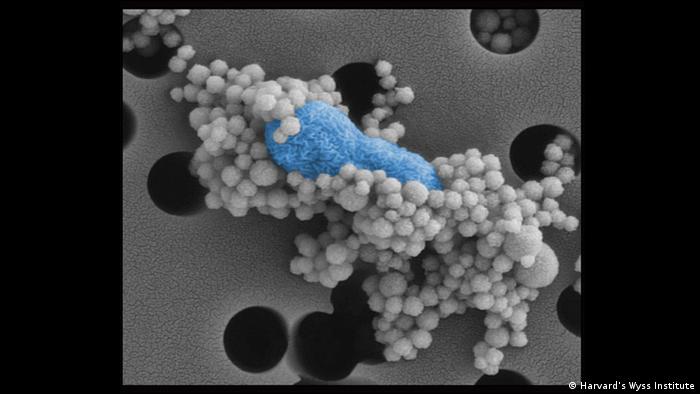
New micromaterial releases nanoparticles that selectively destroy cancer cells
Researchers at the Universitat Autònoma de Barcelona (UAB), in collaboration with the Sant Pau Research Institute and the CIBER-BBN, have developed micromaterials made up only of proteins, capable of delivering over an extended period of time nanoparticles that attack specific cancer cells and destroy them. The micromaterials mimic natural secretory granules found in the endocrine system and were proven effective in mouse models of colorectal cancer.
A team coordinated by Professor Antonio Villaverde from the Institute of Biotechnology and Biomedicine of the Department of Genetics and Microbiology, UAB, and with the participation of the Sant Pau Research Institute and the CIBER-BBN, has developed self-contained micromaterials made up only of proteins that are capable of delivering over an extended period of time the polypeptide that composes them. The technology used for the fabrication of these granules, patented by the researchers, is relatively simple and mimics the secretory granules of the human endocrine system. With regards to its chemical structure, it involves the coordination of ionic zinc with histidine-rich domain, an amino acid essential for living beings and therefore not toxic.
The new micromaterials developed by researchers are formed by chains of amino acids known as polypeptides, which are functional and bioavailable in the form of nanoparticles that can be released and targeted to specific types of cancer cells, for selective destruction.
The research team analyzed the molecular structure of these materials and the dynamics behind the secretion process, both in vitro and in vivo. In an animal model of CXCR4+ colorectal cancer, the system showed high performance upon subcutaneous administration, and how the released protein nanoparticles accumulated in tumor tissues.
“It is important to highlight that this accumulation is more efficient than when the protein is administered in blood. This fact offers an unexpected new way to ensure high local drug levels and better clinical efficacy, thus avoiding repeated intravenous administration regimens”, explains Professor Antonio Villaverde. "In the clinical context, the use of these materials in the treatment of colorectal cancer should largely enhance drug efficiency and patient’s comfort, while at the same time minimizing undesired side effects."
Participating in the research, conducted principally by UAB researcher Julieta M. Sánchez, were researchers from the UAB Department of Genetics and Microbiology, the UAB Institute of Biomedicine and Biotechnology (IBB-UAB), and the Oncogenesis and Antitumor Drugs team led by Professor Ramón Mangues of the Sant Pau Research Institute. Both Professor Antonio Villaverde and Professor Ramón Mangues form part of the CIBER network of Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN). Also participating in the study were the Protein Production Platform (Unit 1) and the Nanotoxicology Platform (Unit 18) of the Singular Infrastructure NANBIOSIS, and funding was received through several competitive research and technology transfer projects (including PID2019 -105416RB-I00/AEI/10.13039/501100011033, PDC2022-133858-I00, PID2022-136845OB-I00, CPP2021-008946, PI21/400), as well as intramural CIBER-BBN projects (VENOM4CANCER, NANOREMOTE and NANOSCAPE).
JOURNAL
Advanced Science
METHOD OF RESEARCH
Experimental study
SUBJECT OF RESEARCH
Animals
ARTICLE TITLE
Structural Stabilization of Clinically Oriented Oligomeric Proteins During their Transit through Synthetic Secretory Amyloids






 “This is an experimental therapy. We don’t have indisputable scientific proof that it will work,” says Doctor Ryszard Międzybrodzki.Kalbar/TFN
“This is an experimental therapy. We don’t have indisputable scientific proof that it will work,” says Doctor Ryszard Międzybrodzki.Kalbar/TFN