Odor-causing bacteria in armpits targeted using bacteriophage-derived lysin
Bacteriophage therapy could be developed based on study’s results
OSAKA METROPOLITAN UNIVERSITY
IMAGE:
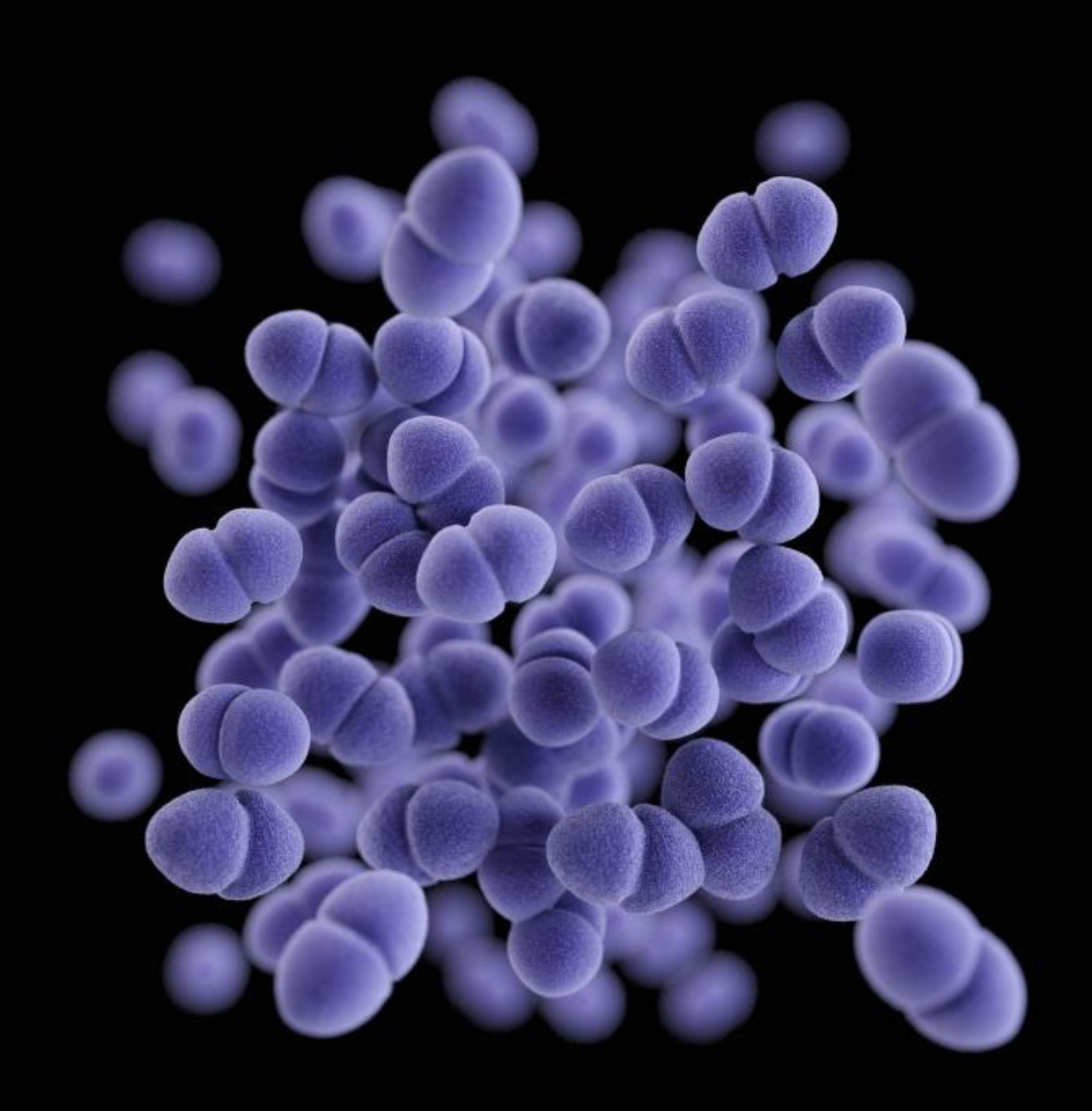
NATIVE BACTERIA METABOLIZE SWEAT IN THE ARMPITS, CAUSING ODOR TO ARISE.
view moreCREDIT: OSAKA METROPOLITAN UNIVERSITY
Body odor from the armpits comes from bacteria metabolizing sweat produced by the apocrine glands. These bacteria are native to our skin, but the odors produced differ among people. Generally, people use deodorants on their armpits, but perhaps there is a way to get rid of the bacteria.
To find out, a research team led by Osaka Metropolitan University Professor Satoshi Uematsu and Associate Professor Kosuke Fujimoto at the Graduate School of Medicine collected body fluid samples from the armpits of 20 men that were deemed healthy. In advance, a subjective olfactory panel classified them into two types of odors, with 11 having a more noticeable smell. The researchers analyzed the matter produced from bacterial metabolism and the DNA of the skin microflora and found an increased presence of odor-causing precursors in those 11 samples along with a proliferation of Staphylococcus hominis bacteria.
The team then synthesized a lysin from a bacteriophage, or virus that attacks bacteria, that infects S. hominis. During in vitro experiments, this lysin was found to target only S. hominis, not other bacteria normally present on the skin.
“We performed a large-scale metagenomic analysis of the skin microflora using the SHIROKANE supercomputer at the University of Tokyo and found that S. hominis is important in the development of odor,” said Assistant Professor Miho Uematsu in the Department of Immunology and Genomics. “The identification of the lysin that attacks S. hominis is also the result of the comprehensive genome analysis.”
Dr. Miki Watanabe, who is part of the Department of Immunology and Genomics and the Department of Dermatology added: “Axillary [armpit] odors are one of the few dermatological disorders in which bacteria are the primary cause. Although many patients suffer from axillary odors, there are few treatment options. We believe that this study will lead to a new therapy.”
The study was published in the Journal of Investigative Dermatology.
###
About OMU
Established in Osaka as one of the largest public universities in Japan, Osaka Metropolitan University is committed to shaping the future of society through “Convergence of Knowledge” and the promotion of world-class research. For more research news, visit https://www.omu.ac.jp/en/ and follow us on social media: X, Facebook, Instagram, LinkedIn.
JOURNAL
Journal of Investigative Dermatology
METHOD OF RESEARCH
Experimental study
SUBJECT OF RESEARCH
People
ARTICLE TITLE
Targeted lysis of Staphylococcus hominis linked to axillary osmidrosis using bacteriophage-derived endolysin
ARTICLE PUBLICATION DATE
19-Apr-2024
COI STATEMENT
Authors declare that they have no competing interests.