SOVIET SCIENCE
Case study highlights the potential—and challenges—of phage therapy
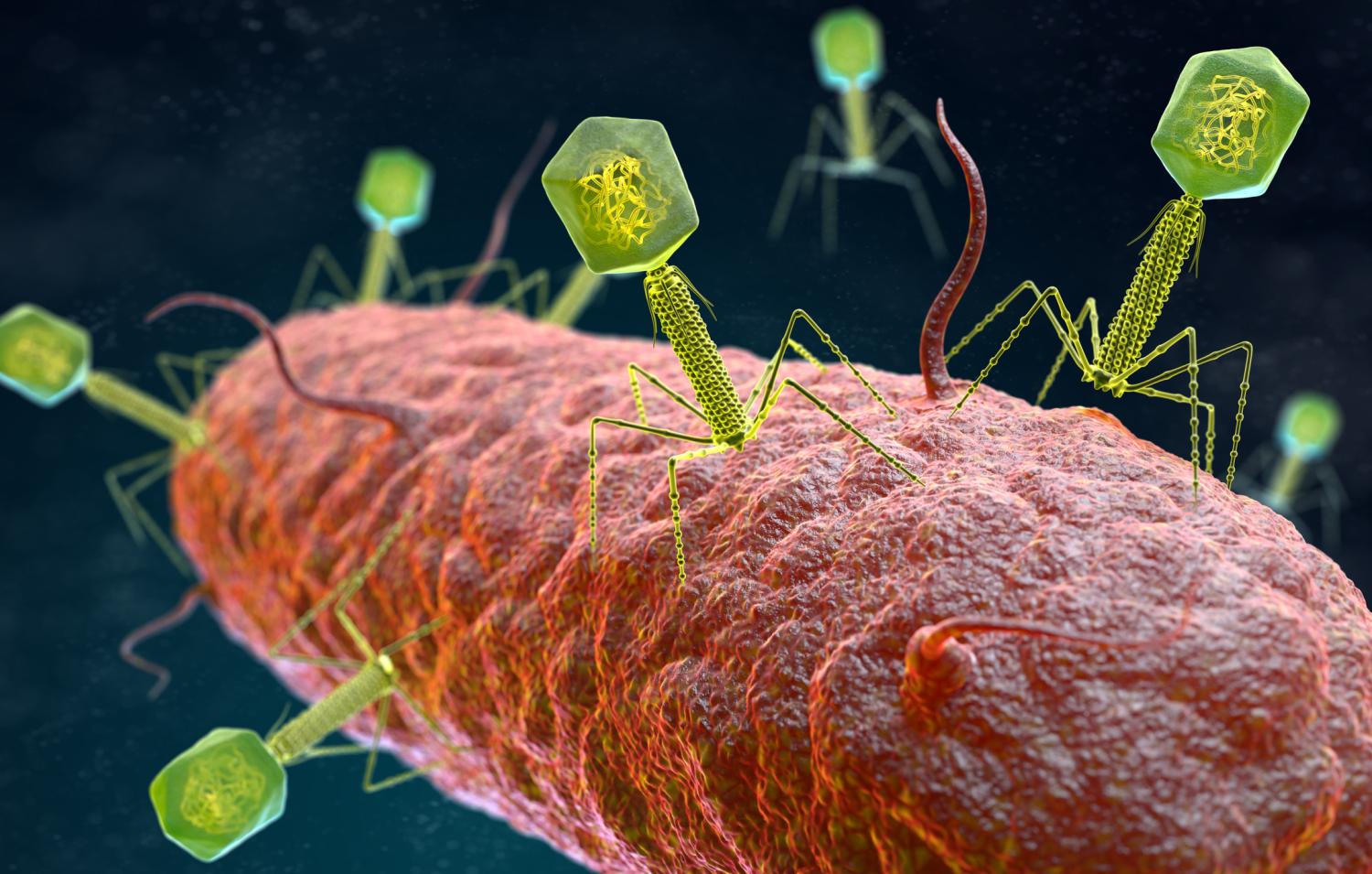
For over two decades, Lynn Cole was in a protracted battle with bacteria and her own immune system.
Diagnosed as having the autoimmune disease Sjogren's syndrome in 1999, Cole suffered from pulmonary fibrosis, was oxygen dependent and highly susceptible to pneumonia, and frequently needed antibiotics for recurrent lung and urinary tract infections. Her daughter, Mya, was there for all of it.
"Most of my childhood was doctor's appointments, inpatient hospital stays, treatments…all that kind of stuff," Mya Cole told CIDRAP News.
But around 2010, Lynn Cole began to have recurrent bloodstream infections caused by the bacterium Enterococcus faecium. From 2013 to 2020, she underwent several hospitalizations at University of Pittsburgh Medical Center (UPMC) for E faecium bloodstream infections and received multiple courses of intravenous antibiotics. At some point in her complex medical history, the bacterium had colonized her gut and become the source of the recurrent infections.
Over that period, Cole, whose case was described in a recent report published in the journal mBio, would typically be sent home with a PICC (peripherally inserted central catheter) line to continue the antibiotic treatment. But within a few days of finishing the antibiotics and removing the PICC line, Cole's blood cultures would be positive for E faecium again.
"We just continued that cycle over and over again, which was frustrating," Mya Cole said.
The cycle continued, with increased frequency, into late 2020, when Cole experienced 26 days of persistent E faecium bacteremia despite treatment with multiple antibiotics that showed in vitro activity against the bacteria.
At that point Cole's treatment team suggested bacteriophages—bacteria-killing viruses—as a potential solution. Cole, after conferring with Mya and her partner, Tina Melotti, said yes.
"We did a little research, and then we talked as a family and agreed that if it could give us a chance, we would try it," Mya said.
A phage cocktail suppresses the infection
To find a phage that might work for Lynn Cole's infection, her doctors turned to researchers at UPMC's Van Tyne lab, which studies how bacteria evolve to resist antibiotics and develops new approaches to treat resistant infections. After receiving the request from Cole's doctors in June 2020, when it had become clear that antibiotics were not going to solve the problem, the lab set out to find a phage that matched the strain of E faecium that was causing the recurrent infections.
Phages aren't hard to find, because they're one of the most abundant organisms on the planet. They can be found in soil, plants, sewage water, and even in the human body. But unlike antibiotics, which work against a narrow or broad spectrum of bacteria, phages have to match the exact strain of bacterium they are targeting to have an effect. That requires testing isolates from a patient's infection to find a match.
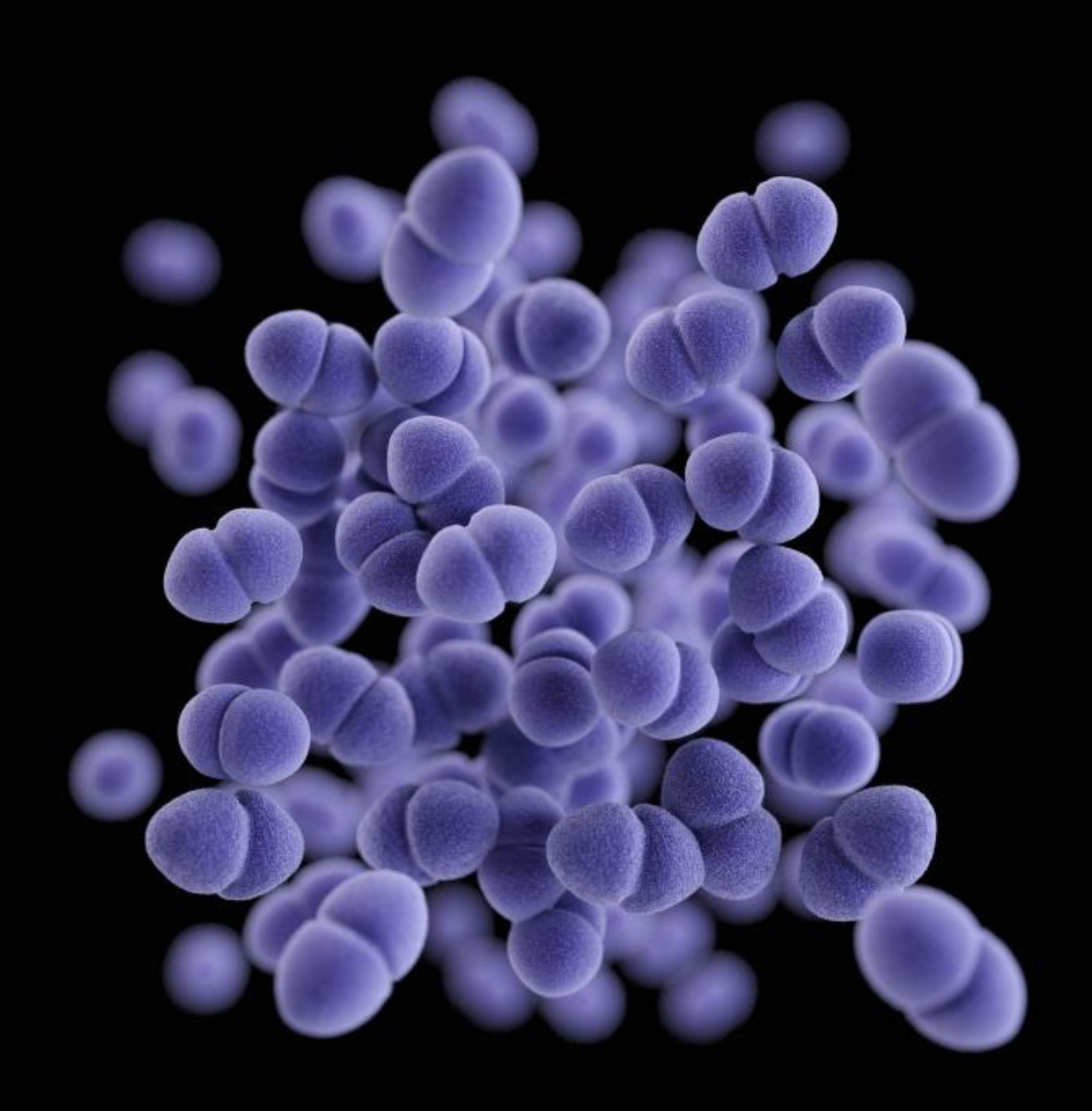
Once a match is found, the identified phage then has to be grown, purified, and prepared for use in a patient. And that's only part of the lengthy process. Because phages are not approved for use in the United States, an Emergency Investigational New Drug (eIND) application for each individual case has to submitted to the US Food and Drug Administration to get the go-ahead.
Ultimately, scientists at the University of Colorado found a phage—9184—that had activity against isolates collected from Cole's infection and sent it the Van Tyne lab, where it was propagated and purified. In December 2020, after spending 20 days in the intensive care unit, Cole began receiving three daily doses of the phage in combination with systemic antibiotics.
"And then, within 24 hours, the blood cultures were clear for the first time that month," said Madison Stellfox, MD, PhD, a member of the Van Tyne lab and co-author of the case report.
After being sent home from the hospital, Cole continued receiving antibiotics and the phage therapy through the PICC line under the supervision of Mya and Tina, both of whom work in healthcare. After a few breakthrough infections that were able to be managed at home, Stellfox and her colleagues added another phage—Hi3—to the treatment regimen.
We did a little research, and then we talked as a family and agreed that if it could give us a chance, we would try it.
Mya Cole
For several months, the phage cocktail appeared to be working. Later analysis of bloodstream isolates and rectal swabs by the Van Tyne lab would show that the abundance of E faecium in Cole's gastrointestinal tract—which the antibiotics alone could not tackle—decreased and remained suppressed when she began receiving the combination of the two phages and the antibiotics.
During that time, Cole was free of the bloodstream infections and able to travel. Her improvement enabled her doctors to step-down the antibiotic and phage regimen. Things were looking up.
"You could definitely tell that she was feeling better," Mya Cole said. "She had a lot more color in her face and a lot more personality."
An unforeseen immune response
If the story ended there, it would add to the list of successful compassionate-use cases whereby phages, in combination with antibiotics, have saved severely ill patients who have multidrug-resistant infections and have run out of options. That success has led to an increase in phage therapy requests.
But that's not where the story ends. On day 395 of her treatment, Cole suffered another E faecium bloodstream infection. At the Van Tyne lab, which had been regularly testing samples of Cole's blood serum that were collected by Mya and Tina to see if the cocktail was still working, they began to see a "precipitous decrease" in phage activity.

When it became clear that the phage therapy was no longer suppressing the infection, Cole and her family decided to cut back on the treatment. She died of pneumonia in 2022, seven-and-a-half months after stopping phage treatment.
While Cole's infection had not become resistant to the phages or the phage-antibiotic combination, Stellfox explains, posthumous analysis of the isolates suggested that the addition of the second phage triggered an immune system response that may have blocked phage activity against the bacteria and resulted in a return of the recurrent bloodstream infections.
"We did see some binding of antibodies to those phages," Stellfox said. "I think that probably played some role."
Case highlights promise, pitfalls
In the paper, Stellfox and her colleagues note that Lynn Cole's experience may not be generalizable to a larger patient population. But she says the case nonetheless highlights both the potential and the pitfalls of phage therapy, which is being increasingly sought out with the emergence and spread of multidrug-resistant bacterial infections and the weak pipeline for new antibiotics.
One major point for her is that phage therapy is safe: The Van Tyne lab has now treated more than 20 patients with phages they've prepared, including 2 others with the same cocktail given to Lynn Cole, and they've seen no severe adverse events.
"I think it shows that if you take the time to do the matchmaking and find that right phage, [phage therapy] can really have a great role in the future," she said.
The challenge of working with phages, however, is that they are not chemicals with set structures, Stellfox noted. And the field lacks the kind of standardized procedures that exist with antibiotics and other approved drugs.
"They're living entities…they adapt, they change, and that's a great thing about them," she said. "But it can also make things trickier."
The immune system is one place where things can get tricky. That's because little is known about what kind of immune response phage therapy will provoke, says Steffanie Strathdee, PhD, co-director of the Center for Innovative Phage Applications and Therapeutics (iPATH) at the University of California, San Diego. For the most part, the focus has been on the interaction between the bacteria and the phage, with the human immune response the "missing part of the triangle."
They're living entities…they adapt, they change, and that's a great thing about them....But it can also make things trickier."
Madison Stellfox, MD, PhD
Strathdee, who co-authored the book The Perfect Predator, which describes her husband's life-threatening Acinetobacter baumannii infection and the phage cocktail that saved him, says that in the compassionate-use cases where phages are needed to save a patient's life, clinicians don't have the luxury of time.
"I don't think it's any surprise that we're going to see cases where antibody is generated against phage," Strathdee said. "But there's no time to say 'hold on, let's assess the patient's immune system to see if there are pre-existing antibodies directed against the phage.' "
Phage therapy 3.0
But just because phages can generate an immune system reaction isn't a reason to "throw out the baby with the bathwater," Strathdee adds, explaining that there have been some cases in which phage therapy has provoked an immune response that wasn't clinically relevant and the patient improved. In addition, she noted, the limitless supply of natural or genetically modified phages means researchers can source new phages that the human immune system hasn't seen yet.
Ultimately, Strathdee believes that what researchers learn from this case and others, along with clinical trials that are under way, will help inform the next stage of phage therapy, or phage therapy 3.0, as she calls it.
"Now we can get smarter about it," she said. "As phage therapy starts to become more mainstream, this issue of the human immune system and its role in phage therapy will become more important."
As phage therapy starts to become more mainstream, this issue of the human immune system and its role in phage therapy will become more important.
Steffanie Strathdee, PhD
Stellfox hopes the case report will help inform future phage research, and says some of the credit should go to Mya and Tina, whose regular collection of blood serum enabled her and her colleagues to get a better understanding of what happened and present their findings.
"They helped us so much, and we are indebted to them," she said.
Mya Cole says that although her mother knew there was no guarantee that phage therapy would cure her or prolong her life, she wanted people to know about and learn from her experience.
"She was very adamant that even though [a cure] couldn't be guaranteed, she wanted her story and her experiences to continue on, even if she did not, so that it could help other patients," she said.
SEE