by Ames Laboratory
Credit: Ames Laboratory
A group of researchers at the U.S. Department of Energy's Ames Laboratory has discovered a way to convert a common byproduct of the paper manufacturing process into valuable chemical precursors for making nylon. The process is much more environmentally friendly in terms of the solvent(s) used and the energy inputs than other methods and provides a useful alternative to burning waste products of pulping.
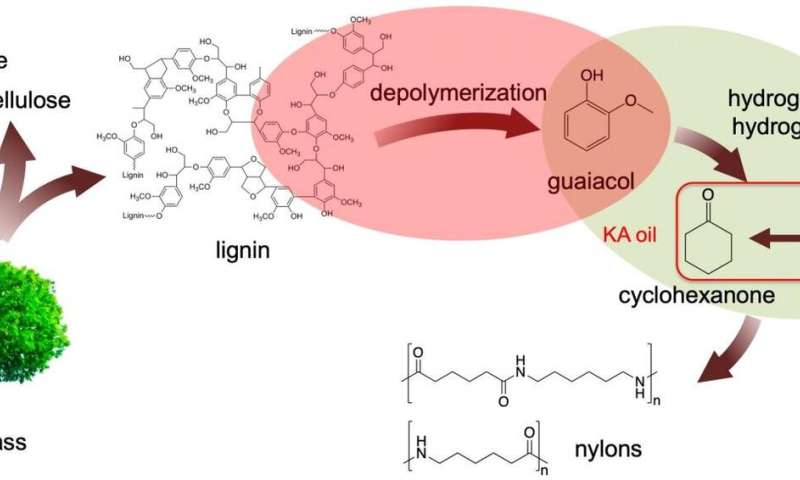
Kraft (from the German meaning strength) lignin is a major waste product of the paper industry, amounting to about 50 million tons annually. This waste lignin is typically burned for heat, however, that process also releases carbon dioxide into the environment.
Ames Laboratory researchers discovered that treating this lignin with aqueous sodium hydroxide at reasonable temperatures (200 °C) produces guaiacol. Guaiacol can then be converted into nylon precursors under even milder conditions using suitable catalysts—creating a new, viable two-step process for producing important chemicals from lignin.
"We found that Kraft lignin was depolymerized in dilute alkaline solution at relatively low temperature (200 °C) under an ambient nitrogen pressure environment," said Igor Slowing, Ames Laboratory scientist and lead investigator. "We were able to produce guaiacol with high selectivity (>80%) in a total monomer amount of 13% based on the lignin input.
The team used a series of techniques including a suite of advanced solution and solid state nuclear magnetic resonance (NMR) experiments, mass spectrometry and model reactions to determine that guaiacol was generated mainly through cleavage of β-O-4 bonds in the original lignin structure. Cleaving this type of bond often requires severe reaction conditions, which many times lead to undesirable side reactions that result in formation of intractable chars."
The Kraft lignin-derived guaiacol was then converted to the nylon precursor Ketone Alcohol or KA oil, using Ru/C catalyst under 1 bar H2. The use of low H2 pressure proved critical to ensure full selectivity to KA oil, without formation of the undesired methoxy-cyclohexanol byproduct. Importantly, the deactivation of the Ru/C catalyst observed in the direct treatment of lignin, was avoided in the two-step procedure.
"This two-step process provides a new option for lignin utilization in the production of high-demand value-added chemicals," said Slowing. "We envision this process as a low-energy path that leaves the remaining oligomers available for downstream processing into other chemical commodities in an integrated refinery for waste Kraft lignin."
The research has been detailed in the Royal Society of Chemistry's journal Green Chemistry, Two-step conversion of Kraft lignin to nylon precursors under mild conditions.
Explore further Transforming waste into bio-based chemicals
A group of researchers at the U.S. Department of Energy's Ames Laboratory has discovered a way to convert a common byproduct of the paper manufacturing process into valuable chemical precursors for making nylon. The process is much more environmentally friendly in terms of the solvent(s) used and the energy inputs than other methods and provides a useful alternative to burning waste products of pulping.
Kraft (from the German meaning strength) lignin is a major waste product of the paper industry, amounting to about 50 million tons annually. This waste lignin is typically burned for heat, however, that process also releases carbon dioxide into the environment.
Ames Laboratory researchers discovered that treating this lignin with aqueous sodium hydroxide at reasonable temperatures (200 °C) produces guaiacol. Guaiacol can then be converted into nylon precursors under even milder conditions using suitable catalysts—creating a new, viable two-step process for producing important chemicals from lignin.
"We found that Kraft lignin was depolymerized in dilute alkaline solution at relatively low temperature (200 °C) under an ambient nitrogen pressure environment," said Igor Slowing, Ames Laboratory scientist and lead investigator. "We were able to produce guaiacol with high selectivity (>80%) in a total monomer amount of 13% based on the lignin input.
The team used a series of techniques including a suite of advanced solution and solid state nuclear magnetic resonance (NMR) experiments, mass spectrometry and model reactions to determine that guaiacol was generated mainly through cleavage of β-O-4 bonds in the original lignin structure. Cleaving this type of bond often requires severe reaction conditions, which many times lead to undesirable side reactions that result in formation of intractable chars."
The Kraft lignin-derived guaiacol was then converted to the nylon precursor Ketone Alcohol or KA oil, using Ru/C catalyst under 1 bar H2. The use of low H2 pressure proved critical to ensure full selectivity to KA oil, without formation of the undesired methoxy-cyclohexanol byproduct. Importantly, the deactivation of the Ru/C catalyst observed in the direct treatment of lignin, was avoided in the two-step procedure.
"This two-step process provides a new option for lignin utilization in the production of high-demand value-added chemicals," said Slowing. "We envision this process as a low-energy path that leaves the remaining oligomers available for downstream processing into other chemical commodities in an integrated refinery for waste Kraft lignin."
The research has been detailed in the Royal Society of Chemistry's journal Green Chemistry, Two-step conversion of Kraft lignin to nylon precursors under mild conditions.
Explore further Transforming waste into bio-based chemicals
More information: Hui Zhou et al. Two-step conversion of Kraft lignin to nylon precursors under mild conditions, Green Chemistry (2020).
Journal information: Green Chemistry
Provided by Ames Laboratory












 This article is republished from
This article is republished from 