Can those who survive COVID-19 provide blood to treat others hospitalized by the disease?
That’s the question driving a Canadian consortium that has launched one of the world’s largest clinical trials of a potential treatment for COVID-19 —one that goes as far back as the Spanish flu a century ago.

Image credit: Pixabay (Free Pixabay license)
The treatment involves taking blood plasma — which contains antibodies — from people who have recovered from COVID-19 infection, and giving it to patients who are sick enough to be hospitalized with the same disease.
The proposed Convalescent Plasma for COVID-19 Research (CONCOR) trial is a collaboration between the Canadian Transfusion Research Network, the McMaster Centre for Transfusion Research, Canadian Blood Services, Héma-Québec and academic partners across the country.
The study is expected to be carried out in every province, and likely each territory. The initial number of people involved is approximately 1,000 patients.
McMaster University professor and hematologist Donald Arnold is leading the trial in conjunction with Philippe Bégin of the University of Montreal and Jeannie Callum of the University of Toronto.
“When people have recovered from COVID-19 infection, we are hoping they will donate a unit of plasma which is essentially the clear portion of blood where all the antibodies are,” said Arnold, an associate professor of medicine and the director of the McMaster Centre for Transfusion Research.
“Presumably those antibodies helped them fight off their COVID-19 infection and allowed them to get better.
The theory is that if you put those antibodies into people who have acute COVID-19 and are in hospital, they may benefit from those antibodies as well.”
The research team’s primary focus is on how the therapy cuts the number of deaths. They will also track a number of other important indicators such as intensive care unit admissions, need for mechanical ventilation, length of stay in hospital or ICU, and side effects from the plasma treatment.
The treatment, called convalescent plasma therapy, has been used during other pandemics, including the Spanish flu of 1918 to 1920.
More recent studies of the use of this treatment during SARS and MERS suggest improved outcomes, but the available evidence is based on low-quality evidence from non-randomized studies.
Arnold notes that within the past week, a publication from China described how convalescent plasma was used to treat COVID-19 patients in intensive care and they recovered, but it was only five patients.
“While there have been reports of people trying this with some success, all of these involved only handfuls of patients and that is all we have to go on,” he said. “We really don’t know if this is truly an effective therapy.”
There are still hurdles before the study can officially commence, including working with the national blood suppliers Canadian Blood Services and Héma-Québec to produce the product.
Arnold hopes for a start date within the next few weeks. Once started, results could be shared, ideally, in three or four months, but more realistically in six to 10 months.
“We’re talking about a clinical trial that would normally take at least six to 12 months to set up,” he says. “We’ve worked out the groundwork in about five days with a national team of committed scientists and physicians.”
Critical to the study’s success is the willingness of Canadians who have recovered from COVID-19 to donate plasma, Arnold says.
“The trial will only succeed if recovering COVID-19 patients are willing to donate their plasma when the time comes,” he said. “There’s a lot of goodwill out there, but this is really pivotal to the trial being successful.”
Arnold said the dedication and commitment demonstrated by partner organizations and academic institutions including the universities of Toronto, Ottawa, Montreal, and British Columbia.
“The most incredible thing about this initiative is how people have come together in such a short period of time with everyone ready to sign on and help in various ways,” he said.
“In only a few days, a wonderful group of top-notch experts across the country has been assembled and they have worked tirelessly to develop this proposal.”
Written by Tina Depko Source: McMaster University
Possible COVID-19 treatment: transfusion of antibodies from recovered patients’ blood
Century-old idea applied to modern pandemic
By Tamara Bhandari March 23, 2020
Possible COVID-19 treatment: transfusion of antibodies from recovered patients’ blood
Century-old idea applied to modern pandemic
By Tamara Bhandari March 23, 2020

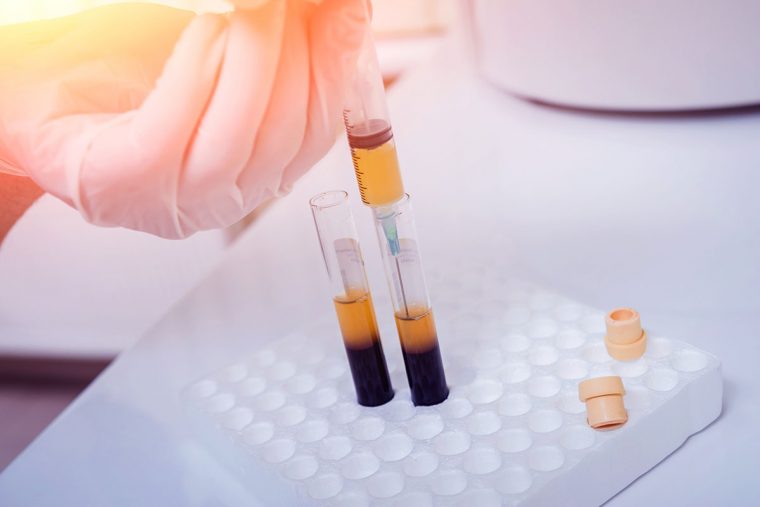
A laboratory worker removes plasma from a vial of blood. Researchers at Washington University School of Medicine in St. Louis and elsewhere are investigating whether transfusions of blood plasma from people who have recovered from COVID-19 can prevent or treat the disease. The approach was used with some success during the 1918 influenza pandemic. (Photo: Getty Images)
With no drugs or vaccines yet approved for COVID-19 and the number of U.S. cases increasing by the thousands every day, doctors are looking to revive a century-old therapy for infectious diseases: transfusing antibodies from the blood of recovered patients into people who are seriously ill.
During the Spanish flu pandemic of 1918, doctors were faced with a deadly illness and no specific treatments. Recognizing that people who had recovered were immune to the infection, some doctors tried treating their patients with blood serum from recovered flu patients. In many cases it worked.
“Giving serum from newly recovered patients is a stone-age approach, but historically it has worked,” said Jeffrey P. Henderson, MD, PhD, associate professor of medicine and of molecular microbiology at Washington University School of Medicine in St. Louis. “This is how we used to prevent and treat viral infections like measles, mumps, polio and influenza, but once vaccines were developed, the technique understandably fell out of favor and many people forgot about it. Until we have specific drugs and vaccines for COVID-19, this approach could save lives.”
Henderson was reminded of the technique by Arturo Casadevall, MD, PhD, the chair of molecular microbiology and immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. Casadevall began championing the idea of using plasma from convalescing patients to treat COVID-19 in early March. Plasma and serum are both the clear fluid portion of blood, and both contain antibodies, but plasma also contains some other proteins lacking in serum.
Plasma transfusion was used experimentally to treat small numbers of people during the SARS outbreak of 2002 and 2003. SARS, which stands for severe acute respiratory syndrome, is caused by a coronavirus closely related to the one that causes COVID-19. In one study, SARS patients who received plasma transfusions recovered faster than those who did not.
Henderson, Casadevall and Michael Joyner, MD, a physiologist at the Mayo Clinic in Rochester, Minn., quickly joined forces and leveraged the resources at their three institutions to test the approach. Their efforts resulted in an investigational new drug application to the Food and Drug Administration that was filed March 18. If the application is approved, they plan to move rapidly to a clinical trial.
“This is something that can be done very quickly, much faster than drug development, because it basically involves donating and transfusing plasma,” Henderson said. “As soon as we have individuals who have recovered from COVID-19 walking around, we have potential donors, and we can use the blood bank system to obtain plasma and distribute it to the patients who need it.”
The plan is to ask patients who recover from COVID-19 to donate their blood, from which plasma would be isolated. After screening for toxins and viruses, the plasma would be transfused into people ill with or at high risk of COVID-19. The procedure for isolating plasma is a long-established technology that can be performed using equipment normally found in blood-banking facilities, and receiving plasma from these donors is as safe as any other plasma transfusion, Henderson said.
The concept is simple, but the execution is more complicated. The scientists still need to determine how much antibody is in the blood of recovered patients, and how much antibody needs to be given to effectively treat or prevent COVID-19. Brenda Grossman, MD, professor of pathology and immunology at Washington University School of Medicine and director of transfusion medicine at Barnes-Jewish Hospital, was brought on board to help navigate the complex regulations surrounding blood donations and transport of blood products across state lines.
The idea is catching fire.
“Last week, it was the three of us on a conference call,” Henderson said. “This week, we had people from all over the country — I don’t even know how many. Everyone’s excited about this. If it works, it could provide a lifeline at this early stage of the pandemic.”
With no drugs or vaccines yet approved for COVID-19 and the number of U.S. cases increasing by the thousands every day, doctors are looking to revive a century-old therapy for infectious diseases: transfusing antibodies from the blood of recovered patients into people who are seriously ill.
During the Spanish flu pandemic of 1918, doctors were faced with a deadly illness and no specific treatments. Recognizing that people who had recovered were immune to the infection, some doctors tried treating their patients with blood serum from recovered flu patients. In many cases it worked.
“Giving serum from newly recovered patients is a stone-age approach, but historically it has worked,” said Jeffrey P. Henderson, MD, PhD, associate professor of medicine and of molecular microbiology at Washington University School of Medicine in St. Louis. “This is how we used to prevent and treat viral infections like measles, mumps, polio and influenza, but once vaccines were developed, the technique understandably fell out of favor and many people forgot about it. Until we have specific drugs and vaccines for COVID-19, this approach could save lives.”
Henderson was reminded of the technique by Arturo Casadevall, MD, PhD, the chair of molecular microbiology and immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. Casadevall began championing the idea of using plasma from convalescing patients to treat COVID-19 in early March. Plasma and serum are both the clear fluid portion of blood, and both contain antibodies, but plasma also contains some other proteins lacking in serum.
Plasma transfusion was used experimentally to treat small numbers of people during the SARS outbreak of 2002 and 2003. SARS, which stands for severe acute respiratory syndrome, is caused by a coronavirus closely related to the one that causes COVID-19. In one study, SARS patients who received plasma transfusions recovered faster than those who did not.
Henderson, Casadevall and Michael Joyner, MD, a physiologist at the Mayo Clinic in Rochester, Minn., quickly joined forces and leveraged the resources at their three institutions to test the approach. Their efforts resulted in an investigational new drug application to the Food and Drug Administration that was filed March 18. If the application is approved, they plan to move rapidly to a clinical trial.
“This is something that can be done very quickly, much faster than drug development, because it basically involves donating and transfusing plasma,” Henderson said. “As soon as we have individuals who have recovered from COVID-19 walking around, we have potential donors, and we can use the blood bank system to obtain plasma and distribute it to the patients who need it.”
The plan is to ask patients who recover from COVID-19 to donate their blood, from which plasma would be isolated. After screening for toxins and viruses, the plasma would be transfused into people ill with or at high risk of COVID-19. The procedure for isolating plasma is a long-established technology that can be performed using equipment normally found in blood-banking facilities, and receiving plasma from these donors is as safe as any other plasma transfusion, Henderson said.
The concept is simple, but the execution is more complicated. The scientists still need to determine how much antibody is in the blood of recovered patients, and how much antibody needs to be given to effectively treat or prevent COVID-19. Brenda Grossman, MD, professor of pathology and immunology at Washington University School of Medicine and director of transfusion medicine at Barnes-Jewish Hospital, was brought on board to help navigate the complex regulations surrounding blood donations and transport of blood products across state lines.
The idea is catching fire.
“Last week, it was the three of us on a conference call,” Henderson said. “This week, we had people from all over the country — I don’t even know how many. Everyone’s excited about this. If it works, it could provide a lifeline at this early stage of the pandemic.”
---30---
No comments:
Post a Comment