A system for the reversible hydrogenation of carbon dioxide into formic acid
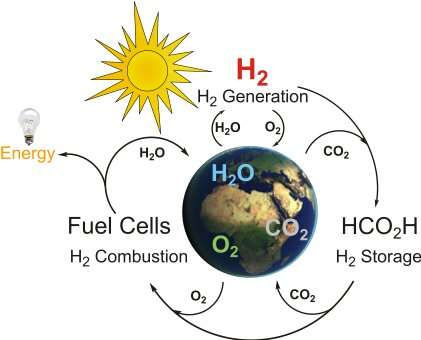
In recent years, engineers have developed a growing number of alternative energy solutions that source electricity sustainably from sunlight, water, wind, hydrogen and other natural resources. For these technologies to fully substitute existing energy solutions, however, the energy they produce will need to be reliably stored and distributed on a large-scale.
Researchers at Leibniz-Institut für Katalyse and APEX Energy Teterow GmbH have recently introduced a new strategy that could aid the storage of chemical energy, particularly hydrogen. In their paper, published in Nature Energy, they outline a system for the reversible hydrogenation of CO2 to formic acid, which employs a Mn-pincer complex as a homogeneous catalyst.
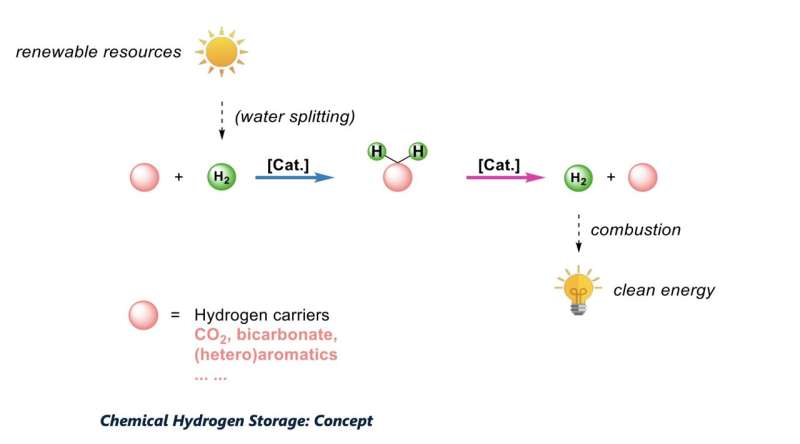
"To transform our current energy system into a more sustainable one, it is important to develop technologies that allow for a practical and efficient storage of renewable energy (wind, photovoltaic, etc.)," Matthias Beller, one of the researchers who carried out the study, told TechXplore. "While the storage of electrons on a large scale is difficult, the storage of chemical energy carriers is easier."
In their previous studies, Beller and his colleagues introduced the idea that formic acid (FA), a simple carboxylic acid that is known to be contained in bee venom and other natural materials, could be a good hydrogen carrier. They showed that FA can be generated from CO2, as well as what is known as "green hydrogen" (i.e., hydrogen produced by splitting water intro hydrogen and oxygen using renewable energy technology).
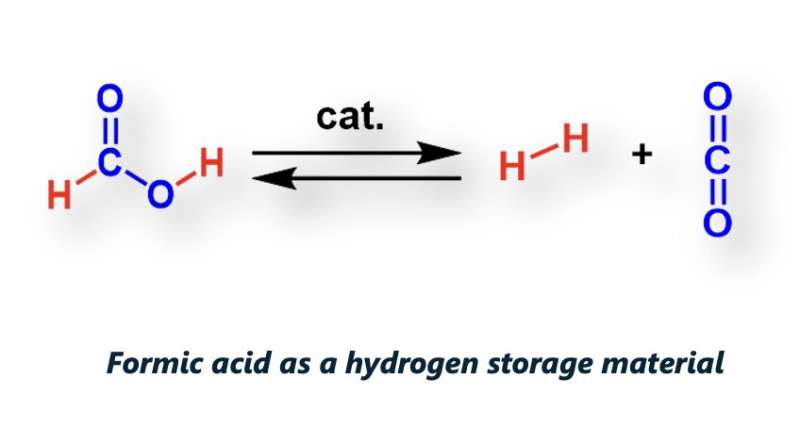
"If energy is needed FA can be easily dehydrogenated under mild conditions and provides electricity on demand in well-established PEM fuel cells," Beller explained. "Parallel to the release of hydrogen, normally CO2 is also released, due to its gaseous nature. Hence, when you want to generate the hydrogen carrier back, you need carbon dioxide again."
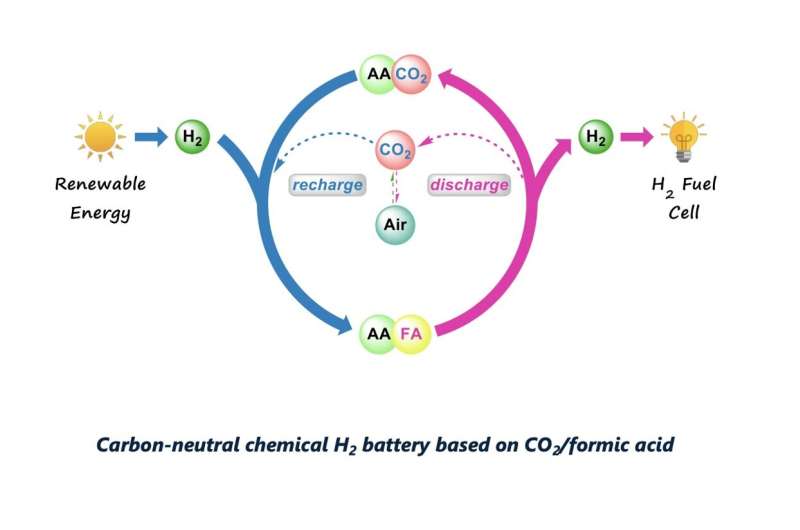
The new system for the hydrogenation of CO2 introduced by Beller, Henrik Junge, Peter Sponholz and their colleagues, does not require CO2 again once the first charging process is completed. In fact, its design ensures that the CO2 remains in the reaction medium, which eliminates the need for additional CO2.
The researchers' system relies on the use of a Manganese (Mn)-based pincer complex and L-lysine, an essential α-amino acid.
"We were surprised to find that the combination L-lysine and Mn pincer catalysts allows for hydrogenation of CO2 in the air with extremely high efficiency," Beller said. "In this process, initially the amino functions of L-lysine bind to CO2 , forming as so-called carbamic acid derivative, which is in equilibrium with the corresponding bicarbonate. Hydrogenation of the activated CO2 leads to the formation of FA, and by lowering the pressure hydrogen can be released from the system."
The researchers evaluated their system in a series of tests and showed that it attained highly promising results. Notably, they found that both the Mn catalyst and L-lysine had a good stability and could be used again numerous times. Overall, their system attained a total turnover number of 2,000,000 for CO2 hydrogenation and of 600,000 for FA dehydrogenation.
When they used potassium lysinate, the researchers achieved an H2 evolution efficiency above 80% and a CO2 retention of over 99.9% over ten charge and discharge cycles, without having to re-load CO2 between these cycles. The team also found that this reversible hydrogenation process could be scaled up considerably, without significantly reducing the system's productivity.
"An interesting finding of our work is the analogy between hydrogen storage systems and traditional electric batteries," Beller said. "In a typical rechargeable battery, electrons can be added and released under very mild (ambient) conditions. In principle, the same is true for hydrogen storage materials if the reaction system is designed in an appropriate manner."
In the future, the system for the reversible hydrogenation of CO2 to FA introduced by this team of researchers could help to store green hydrogen more efficiently. This could contribute to the large-scale implementation of fuel cells and other hydrogen-based sustainable technologies.
"For the 'hydrogen battery,' we now would like to improve the energy content of the system by generating methanol instead of FA," Beller added. "In addition, we want to improve the practicability of such a system by performing charging/decharging steps in an automatic manner."
New biobattery for hydrogen storage
More information: Duo Wei et al, Reversible hydrogenation of carbon dioxide to formic acid using a Mn-pincer complex in the presence of lysine, Nature Energy (2022). DOI: 10.1038/s41560-022-01019-4
Journal information: Nature Energy
© 2022 Science X Network
No comments:
Post a Comment