Exclusive: ‘People on antidepressants long-term say they feel blunted, with psychedelic therapy it’s the opposite, they talk about an emotional release, a reconnection’
Alex Matthews-King Health Correspondent
Hallucination-inducing drugs like magic mushrooms could be about to break big pharma’s stranglehold on the hugely lucrative market for antidepressants, according to the head of the world’s first centre for psychedelic research.
Antidepressant prescriptions have doubled in England in a decade with around seven million adults taking the drugs, and the global market is predicted to be worth $15.9bn (£12.5bn) by 2023.
At Imperial College London, Dr Robin Carhart-Harris is leading one of the first trials to test how therapy using psilocybin mushrooms, which are currently banned in the UK, compares to leading antidepressants.
While he won’t prejudge the results of the study, he says participants describe a cathartic emotional “release” with psilocybin therapy – the polar opposite of antidepressants, which patients complain leave their emotions, whether positive or negative, “blunted”.
It is the first of many studies planned under the banner of the new Centre for Psychedelic Research at London’s Imperial College.
Read more

The medicinal wonders of psychedelics are finally being recognised
A large empty floor of the university’s Hammersmith campus will house a bank of treatment rooms that make it the UK’s first psychedelic therapy research clinic, and a “prototype and inspiration” for licensed psychedelic medicine clinics of the future.
Trials of psilocybin in treating eating disorders, and a study of the effects of powerful hallucinogenic DMT on the brain, are already planned following Imperial’s commitment to the centre.

The future home of the Psychedelic Research Centre treatment rooms which could become the model for future clinics (The Independent)
But it is the work on depression where research is most advanced, and most promising.
On the current trial, around 60 participants with moderate to severe depression will receive psilocybin treatment accompanied by a therapy session with a clinical psychologist.
The participants will also be randomly allocated to receive either a placebo or the drug escitalopram, with neither researchers or patients knowing who is in each group.

Magic mushrooms could be bought in the UK until 2005 (Getty)
Escitalopram is a type of selective serotonin reuptake inhibitors (SSRIs), the drugs which account for the largest chunk of the antidepressant market.
“If you ask people who are taking SSRIs chronically, they often say ‘I feel blunted’,” Dr Carhart-Harris told The Independent, meaning both negative and positive emotions are suppressed.
“With psilocybin therapy they say the opposite, they talk about an emotional release, a reconnection, and this key emotional centre being more responsive.”
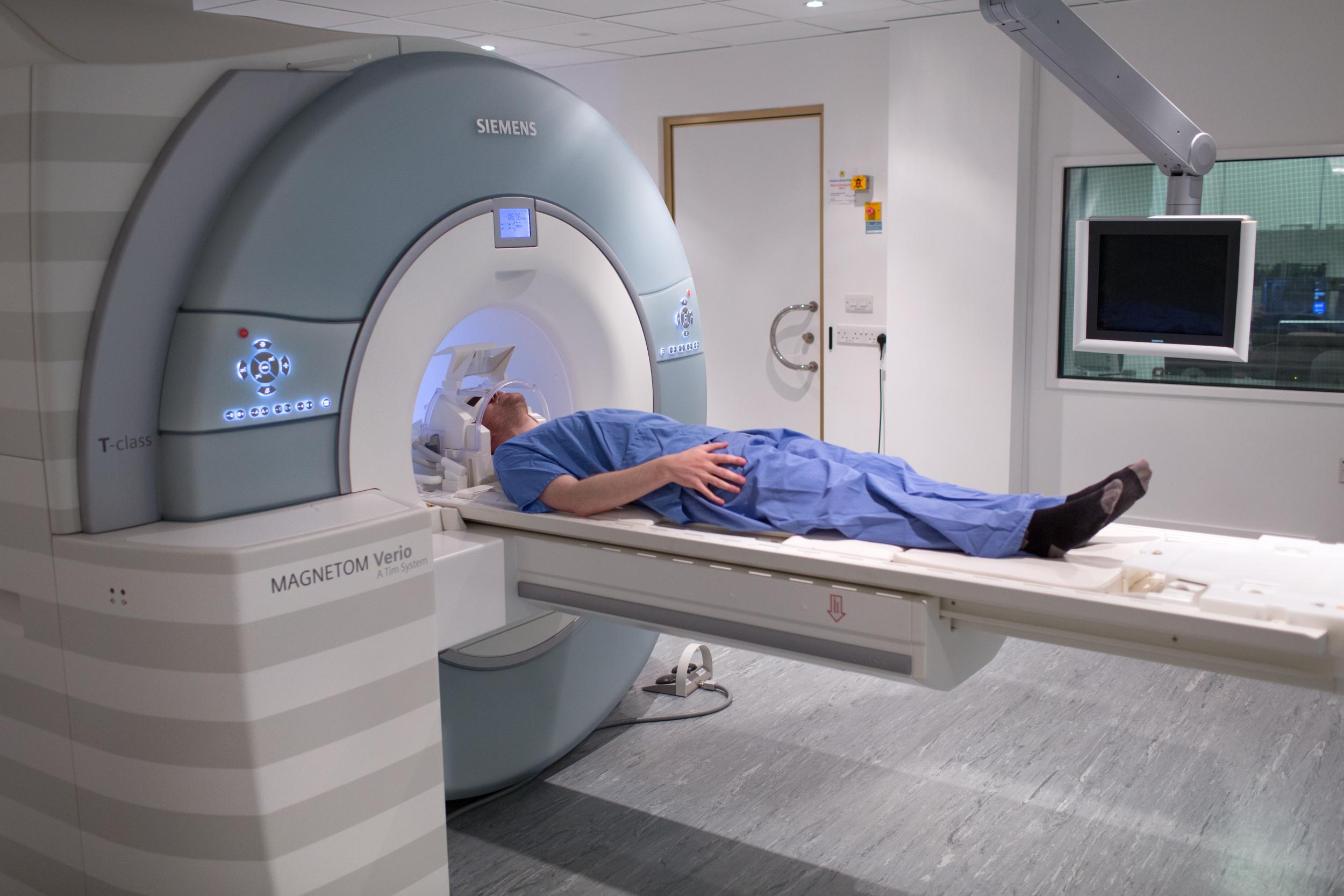
Patients will have MRI scans to test changes in their brains after
psilocybin therapy (Centre for Psychedelic Research)
The team use MRI scans to study psychedelics’ effects on the brain and the drug appears to reduce activity in the coordinating regions, releasing their grip and allowing the more primitive emotional centres to the fore.
Other early indications are that the list of side-effects is “twice as long” for escitalopram as it is for psilocybin therapy, and it is much faster acting than antidepressants – which can take months to work.

Treatment rooms are a soothing environment where participants are
supported through their psychedelic experience (Centre for Psychedelic Research)
However, the treatment may not be suitable for everyone.
During the therapy sessions, patients are encouraged to follow the stream of the psychedelic experience which can be extremely vivid and may require them to confront past traumas or experiences.
“We don’t call it a ‘bad trip’,” Dr Carhart-Harris says. “We call it a ‘challenging psychological experience’ and we’re honest with people that it can be hellish.
“It can be nightmarish, but we’re prepared for this and this treatment model requires you literally face your demons.”
Psychedelic therapy is unlikely to be suitable for people with psychosis and regulators will need evidence of its effectiveness and safety from clinical trials.
But there is little evidence that they pose a risk of overdose or addiction and that could speed their route to approval.
Fresh magic mushrooms could be picked or bought in shops legally in the UK until 2005, when a change in law closed the loop hole and made them Class A drugs alongside crack cocaine.
“I would imagine if you had some bookmakers doing the odds, there would be strong odds on that [psychedelic therapy] will be licensed sometime in the next five to 10 years – maybe sooner,” Dr Carhart-Harris says.
That could put it on a collision course with powerful interests of the pharmaceutical industry, particularly if trials show psilocybin therapy to be superior to SSRIs
Psilocybin therapy may allow depressed people’s brains to rewire in
a positive way, rather than suppressing good and bad emotion (Centre for Psychedelic Research)
“The implications of that are actually frightening to me, thinking of the power and influence of big pharma,” Carhart-Harris says. “What are they going to do with that if there’s this big public demand for the ‘mushroom therapy’, and not the Prozac?”
While there is a growing trend for “microdosing” psilocybin or LSD, the evidence to date suggests it is the combination of therapy and psychedelic experience that offers the best option of a lasting alternative to chronic antidepressants.

Psychedelics research has had to rely on philanthropic funding for years,
but latest wave of trials show the field is set on not repeating the mistakes
of the past (The Independent)
“If you strip the drug away from therapy you start seeing the adverse events that were being reported in the 1960s, when psychedelics left the clinic and became popularised,” Dr Carhart-Harris adds.
“None of us want those mistakes to be made again.”
Dr James Rucker is another of those researching the potential benefits of psychedelics, over at the Institute of Psychiatry, Psychology and Neuroscience at King’s College London.
The King’s team are launching two trials, one looking at whether psilocybin therapy can help people whose depression is resistant to treatment with conventional antidepressants.
He says it was “possible” the drug could be licensed in five years. “But only if everything goes to plan, and you know what they say about best-laid plans.”
In Dr Rucker’s mind the process is similar to the approval of ketamine, where the first trials in depression took place in the 1990s and the first ketamine-based medicines are only now being licensed.
Psilocybin has much lower potential for abuse and overdose, but watchdogs will still need stage three trials which haven’t even begun.
“Like all treatments, they will suit some people but not others,” he told The Independent. “The trick, as ever, is trying to work that out before administration. But that trick has proven to be remarkably difficult to pull off, particularly in psychiatry.
Psychedelics research has had to rely on philanthropic funding for years, but latest wave of trials show the field is set on not repeating the mistakes of the past (The Independent)
“If you strip the drug away from therapy you start seeing the adverse events that were being reported in the 1960s, when psychedelics left the clinic and became popularised,” Dr Carhart-Harris adds.
“None of us want those mistakes to be made again.”
Dr James Rucker is another of those researching the potential benefits of psychedelics, over at the Institute of Psychiatry, Psychology and Neuroscience at King’s College London.
The King’s team are launching two trials, one looking at whether psilocybin therapy can help people whose depression is resistant to treatment with conventional antidepressants.
He says it was “possible” the drug could be licensed in five years. “But only if everything goes to plan, and you know what they say about best-laid plans.”
In Dr Rucker’s mind the process is similar to the approval of ketamine, where the first trials in depression took place in the 1990s and the first ketamine-based medicines are only now being licensed.
Psilocybin has much lower potential for abuse and overdose, but watchdogs will still need stage three trials which haven’t even begun.
“Like all treatments, they will suit some people but not others,” he told The Independent. “The trick, as ever, is trying to work that out before administration. But that trick has proven to be remarkably difficult to pull off, particularly in psychiatry.”
FOR ARTICLE WITH VIDEO'S AND PICTURES GO HERE
A large empty floor of the university’s Hammersmith campus will house a bank of treatment rooms that make it the UK’s first psychedelic therapy research clinic, and a “prototype and inspiration” for licensed psychedelic medicine clinics of the future.
Trials of psilocybin in treating eating disorders, and a study of the effects of powerful hallucinogenic DMT on the brain, are already planned following Imperial’s commitment to the centre.

The future home of the Psychedelic Research Centre treatment rooms which could become the model for future clinics (The Independent)
But it is the work on depression where research is most advanced, and most promising.
On the current trial, around 60 participants with moderate to severe depression will receive psilocybin treatment accompanied by a therapy session with a clinical psychologist.
The participants will also be randomly allocated to receive either a placebo or the drug escitalopram, with neither researchers or patients knowing who is in each group.

Magic mushrooms could be bought in the UK until 2005 (Getty)
Escitalopram is a type of selective serotonin reuptake inhibitors (SSRIs), the drugs which account for the largest chunk of the antidepressant market.
“If you ask people who are taking SSRIs chronically, they often say ‘I feel blunted’,” Dr Carhart-Harris told The Independent, meaning both negative and positive emotions are suppressed.
“With psilocybin therapy they say the opposite, they talk about an emotional release, a reconnection, and this key emotional centre being more responsive.”

Patients will have MRI scans to test changes in their brains after
psilocybin therapy (Centre for Psychedelic Research)
The team use MRI scans to study psychedelics’ effects on the brain and the drug appears to reduce activity in the coordinating regions, releasing their grip and allowing the more primitive emotional centres to the fore.
Other early indications are that the list of side-effects is “twice as long” for escitalopram as it is for psilocybin therapy, and it is much faster acting than antidepressants – which can take months to work.

Treatment rooms are a soothing environment where participants are
supported through their psychedelic experience (Centre for Psychedelic Research)
However, the treatment may not be suitable for everyone.
During the therapy sessions, patients are encouraged to follow the stream of the psychedelic experience which can be extremely vivid and may require them to confront past traumas or experiences.
“We don’t call it a ‘bad trip’,” Dr Carhart-Harris says. “We call it a ‘challenging psychological experience’ and we’re honest with people that it can be hellish.
“It can be nightmarish, but we’re prepared for this and this treatment model requires you literally face your demons.”
Psychedelic therapy is unlikely to be suitable for people with psychosis and regulators will need evidence of its effectiveness and safety from clinical trials.
But there is little evidence that they pose a risk of overdose or addiction and that could speed their route to approval.
Fresh magic mushrooms could be picked or bought in shops legally in the UK until 2005, when a change in law closed the loop hole and made them Class A drugs alongside crack cocaine.
“I would imagine if you had some bookmakers doing the odds, there would be strong odds on that [psychedelic therapy] will be licensed sometime in the next five to 10 years – maybe sooner,” Dr Carhart-Harris says.
That could put it on a collision course with powerful interests of the pharmaceutical industry, particularly if trials show psilocybin therapy to be superior to SSRIs

Psilocybin therapy may allow depressed people’s brains to rewire in
a positive way, rather than suppressing good and bad emotion (Centre for Psychedelic Research)
“The implications of that are actually frightening to me, thinking of the power and influence of big pharma,” Carhart-Harris says. “What are they going to do with that if there’s this big public demand for the ‘mushroom therapy’, and not the Prozac?”
While there is a growing trend for “microdosing” psilocybin or LSD, the evidence to date suggests it is the combination of therapy and psychedelic experience that offers the best option of a lasting alternative to chronic antidepressants.

Psychedelics research has had to rely on philanthropic funding for years,
but latest wave of trials show the field is set on not repeating the mistakes
of the past (The Independent)
“If you strip the drug away from therapy you start seeing the adverse events that were being reported in the 1960s, when psychedelics left the clinic and became popularised,” Dr Carhart-Harris adds.
“None of us want those mistakes to be made again.”
Dr James Rucker is another of those researching the potential benefits of psychedelics, over at the Institute of Psychiatry, Psychology and Neuroscience at King’s College London.
The King’s team are launching two trials, one looking at whether psilocybin therapy can help people whose depression is resistant to treatment with conventional antidepressants.
He says it was “possible” the drug could be licensed in five years. “But only if everything goes to plan, and you know what they say about best-laid plans.”
In Dr Rucker’s mind the process is similar to the approval of ketamine, where the first trials in depression took place in the 1990s and the first ketamine-based medicines are only now being licensed.
Psilocybin has much lower potential for abuse and overdose, but watchdogs will still need stage three trials which haven’t even begun.
“Like all treatments, they will suit some people but not others,” he told The Independent. “The trick, as ever, is trying to work that out before administration. But that trick has proven to be remarkably difficult to pull off, particularly in psychiatry.
Psychedelics research has had to rely on philanthropic funding for years, but latest wave of trials show the field is set on not repeating the mistakes of the past (The Independent)
“If you strip the drug away from therapy you start seeing the adverse events that were being reported in the 1960s, when psychedelics left the clinic and became popularised,” Dr Carhart-Harris adds.
“None of us want those mistakes to be made again.”
Dr James Rucker is another of those researching the potential benefits of psychedelics, over at the Institute of Psychiatry, Psychology and Neuroscience at King’s College London.
The King’s team are launching two trials, one looking at whether psilocybin therapy can help people whose depression is resistant to treatment with conventional antidepressants.
He says it was “possible” the drug could be licensed in five years. “But only if everything goes to plan, and you know what they say about best-laid plans.”
In Dr Rucker’s mind the process is similar to the approval of ketamine, where the first trials in depression took place in the 1990s and the first ketamine-based medicines are only now being licensed.
Psilocybin has much lower potential for abuse and overdose, but watchdogs will still need stage three trials which haven’t even begun.
“Like all treatments, they will suit some people but not others,” he told The Independent. “The trick, as ever, is trying to work that out before administration. But that trick has proven to be remarkably difficult to pull off, particularly in psychiatry.”
FOR ARTICLE WITH VIDEO'S AND PICTURES GO HERE
No comments:
Post a Comment