New paper explores how quantum computing can unlock understanding of aging and disease
IMAGE:
BIOLOGICAL NETWORKS ARE INTERCONNECTED. JUST AS KNOWING THE INGREDIENTS ALONE IS NOT SUFFICIENT TO UNDERSTAND HOW TO PREPARE A DISH, UNDERSTANDING ONLY THE LIST OF GENES OR PROTEINS IS INSUFFICIENT TO COMPREHEND HOW THEY INTERACT.
view moreCREDIT: INSILICO MEDICINE
In a new paper in WIREs Computational Molecular Science, researchers from clinical stage artificial intelligence (AI)-driven drug discovery company Insilico Medicine (“Insilico”) demonstrate how quantum computing can be integrated into the study of living organisms in order to provide greater insight into biological processes like aging and disease.
In May 2023, Insilico, University of Toronto’s Acceleration Consortium, and Foxconn Research Institute published research that successfully demonstrated the potential advantages of quantum generative adversarial networks in generative chemistry. Those findings were published in the American Chemical Society’s Journal of Chemical Information and Modeling.
In this latest paper, Insilico researchers present a broad picture of how combining methods from AI, quantum computing, and the physics of complex systems can help researchers advance new understandings of human health – and detail the latest breakthroughs in physics-guided AI.
While AI has been an invaluable tool in helping researchers process and analyze large, complex biological datasets in order to find new disease pathways and connect aging and disease at the cellular level, they write, it still faces challenges in applying those insights to more complex interactions within the body.
In order to fully understand the inner workings of living organisms, the researchers note, scientists need multimodal modeling methods that can manage three key areas of complexity: the complexity of scale, the complexity of the algorithms, and the increasing complexity of datasets.
“While we are not a quantum company, it is important to utilize capabilities to take advantage of the speed provided by the new hybrid computing solutions and hyperscalers. As this computing goes mainstream, it may be possible to perform very complex biological simulations and discover personalized interventions with desired properties for a broad range of diseases and age-associated processes. We are very happy to see our research center in the UAE producing valuable insights in this area,” says co-author Alex Zhavoronkov, PhD, founder and co-CEO of Insilico Medicine.
Biological processes within living systems scale from cells to organs to the whole body with lots of complex interactions between systems. Interpreting these processes needs to work on multiple scales simultaneously. And access to biological data has reached previously unimaginable levels. There’s the 1000 Genomes Project – a catalog of human genetic variation which has identified over 9 million single nucleotide variants (SNVs) – and the UK Biobank which contains full sequences from 500,000 genomes of British volunteers, to name just a couple. We need massive computing power to analyze and process it.
Quantum computing, the researchers write, is uniquely positioned to augment AI approaches – allowing researchers to interpret across multiple levels of the biological system simultaneously. Because qubits hold values of 0 and 1 simultaneously, whereas classical bits hold only values of 0 or 1, qubits have massively greater computing speed and capability.
The authors note that major advances in quantum computing are already underway, including IBM’s recent debut of both a utility-scale quantum processor and the company’s first modular quantum computer, which has already begun operations.
Ultimately, the authors call for a physics-guided AI approach to better understand human biology – a new field that combines physics-based and neural network models, which they write is already underway.
By combining methods from AI, quantum computing, and the physics of complex systems, scientists can better understand how, as the authors write, “the collective interactions of smaller-scale elements within a cell, organism, or society generate emergent characteristics that can be observed at larger scales and levels of reality.”
Hierarchical complexity of living organisms 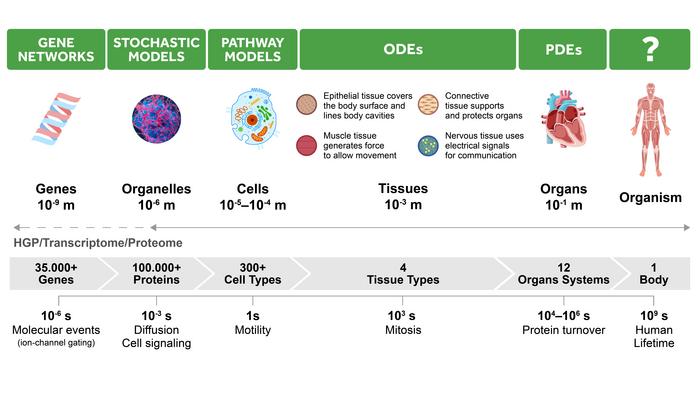
CAPTION
At each hierarchical scale, there is a most-used method for studying this level of organization. AI shows potential at each of the levels. Quantum computing provides possibilities for speed-up and increased effectiveness of both AI solvers and traditional techniques.
CREDIT
Insilico Medicine
About Insilico Medicine
Insilico Medicine, a global clinical stage biotechnology company powered by generative AI, is connecting biology, chemistry, and clinical trials analysis using next-generation AI systems. The company has developed AI platforms that utilize deep generative models, reinforcement learning, transformers, and other modern machine learning techniques for novel target discovery and the generation of novel molecular structures with desired properties. Insilico Medicine is developing breakthrough solutions to discover and develop innovative drugs for cancer, fibrosis, immunity, central nervous system diseases, infectious diseases, autoimmune diseases, and aging-related diseases. www.insilico.com
The Conversation
January 26, 2024

Brain (Shutterstock)
Human behavior is an enigma that fascinates many scientists. And there has been much discussion over the role of probability in explaining how our minds work.
Probability is a mathematical framework designed to tell us how likely an event is to occur – and works well for many everyday situations. For example, it describes the outcome of a coin toss as ½ – or 50% – because throwing either heads or tails is equally probable.
Yet research has shown that human behavior can’t be fully captured by these traditional or “classical” laws of probability. Could it instead be explained by the way probability works in the more mysterious world of quantum mechanics?
Mathematical probability is also a vital component of quantum mechanics, the branch of physics that describes how nature behaves at the scale of atoms or sub-atomic particles. However, as we’ll see, in the quantum world, probabilities follow very different rules.
Discoveries over the last two decades have shed light on a crucial role for “quantumness” in human cognition – how the human brain processes information to acquire knowledge or understanding. These findings also have potential implications for the development of artificial intelligence (AI).
Nobel laureate Daniel Kahnemann and other cognitive scientists have carried out work on what they describe as the “irrationality” of human behavior. When behavioral patterns do not strictly follow the rules of classical probability theory from a mathematical perspective, they are deemed “irrational”.
For example, a study found that a majority of students who have passed an end-of-term exam favor going on holiday afterwards. Likewise, a majority of those who have failed also want to go for a holiday.
If a student doesn’t know their result, classical probability would predict that they would opt for the holiday because it is the preferred option whether they have passed or failed. Yet in the experiment, a majority of students preferred not to go on holiday if they didn’t know how they’d done.
Intuitively, it’s not hard to understand that students might not want to go on holiday if they are going to be worrying about their exam results the whole time. But classical probability does not accurately capture the behavior, so it is described as irrational. Many similar violations of classical probability rules have been observed in cognitive science.
Quantum brain?
In classical probability, when a sequence of questions is asked, then the answers do not depend on the order in which the questions are posed. By contrast, in quantum physics, the answers to a series of questions can depend crucially on the order in which they are asked.
One example is the measurement of the spin of an electron in two different directions. If you first measure the spin in the horizontal direction and then in the vertical direction, you will get one outcome.
The outcomes will generally be different when the order is reversed, because of a well known feature of quantum mechanics. Simply measuring a property of a quantum system can affect the thing that’s being measured (in this case an electron’s spin) and hence the outcome of any subsequent experiments.
Order dependence can also be seen in human behavior. For example, in a study published 20 years ago about the effects that question order has on respondents’ answers, subjects were asked whether they thought the previous US president, Bill Clinton, was honest.
When the questions were delivered in this order, a respective 50% and 60% of respondents answered that they were honest. But when the researchers asked respondents about Gore first and then Clinton, a respective 68% and 60% responded that they were honest.
On an everyday level, it might seem that human behavior is not consistent because it often violates the rules of classical probability theory. However, this behaviour does appear to fit with the way probability works in quantum mechanics.
Observations of this kind have led cognitive scientist Jerome Busemeyer and many others to recognize that quantum mechanics can, on the whole, explain human behavior in a more consistent way.
Based on this astonishing hypothesis, a new research field called “quantum cognition” has arisen within the area of cognitive sciences.
How it is possible that thought processes are dictated by quantum rules? Is our brain working like a quantum computer? No one yet knows the answers, but the empirical data strongly appears to suggest that our thoughts follow quantum rules.
Dynamic behaviour
In parallel to these exciting developments, over the past two decades my collaborators and I have developed a framework for modeling – or simulating – the dynamics of people’s cognitive behavior as they digest “noisy” (that is, imperfect) information from the outside world.
We again found that mathematical techniques developed for modelling the quantum world could be applied to modeling how the human brain processes noisy data.
These principles can be applied to other behavior in biology, beyond just the brain. Green plants, for example, have the remarkable ability to extract and analyse chemical and other information from their environments and to adapt to changes.
My rough estimate, based on a recent experiment on common bean plants, suggests that they can process this external information more efficiently than the best computer we have today.
In this context, efficiency means that the plant is consistently able to reduce the uncertainty about its external environment to the greatest extent possible in its circumstances. This could, for example, encompass easily detecting the direction that light is coming from, so that the plant can grow towards it. The efficient processing of information by an organism is also linked to saving energy, which is important for its survival.
Similar rules may apply to the human brain, particularly to how our state of mind changes when detecting outside signals. All of this is important for the current trajectory of technological development. If our behavior is best described by the way probability works in quantum mechanics, then to accurately replicate human behavior in machines, AI systems should probably follow quantum rules, not classical ones.
I’ve called this idea artificial quantum intelligence (AQI). A great deal of research is needed to develop practical applications from such an idea.
But an AQI could help get us to the goal of AI systems that behave more like a real person.

Dorje C. Brody, Professor of Mathematics, University of Surre
An electrolyte additive increased the charging rate of lithium metal batteries and led to new discoveries about battery chemistry
IMAGE:
FROM LEFT TO RIGHT: BROOKHAVEN BEAMLINE SCIENTIST SANJIT GHOSE WITH CHEMISTS ENYUAN HU AND MUHAMMAD MOMINUR RAHMAN AT THE NATIONAL SYNCHROTRON LIGHT SOURCE II X-RAY POWDER DIFFRACTION BEAMLINE.
view moreCREDIT: (JESSICA ROTKIEWICZ/BROOKHAVEN NATIONAL LABORATORY)
UPTON, NY—On a mission to build better electric vehicle batteries, chemists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory have used an electrolyte additive to improve the functionality of energy-dense lithium metal batteries. By adding a compound called cesium nitrate to the electrolyte that separates the battery’s anode and cathode, the research team has significantly improved the charging rate of lithium metal batteries while maintaining a long cycle life.
The team’s new work, recently published in Nature Communications, targets the interphase—a protective layer formed on the battery’s anode and cathode. This layer, which prevents degradation of battery electrodes, is the key to creating lithium metal batteries that can be charged and discharged as many times as lithium-ion batteries.
“We wanted to improve the charging rate of the current state-of-the-art lithium metal batteries,” explained Muhammad Mominur Rahman, a research associate in the Electrochemical Energy Storage Group of the Chemistry Division at Brookhaven and first author on the new paper. “But we also wanted to stabilize the batteries with a more protective interphase so they would last longer.”
In addition to successfully stabilizing the battery, Rahman’s electrolyte additive altered the battery chemistry in an unexpected way.
“Mominur’s findings challenge conventional beliefs about the components of an effective interphase,” said Enyuan Hu, Brookhaven chemist and principal investigator within the Electrochemical Energy Storage Group. “We’re excited to see how these findings contribute to the major DOE effort focused on lithium metal batteries.”
One step towards a larger goal
Hu and his team are working among other battery experts as part of the Battery500 Consortium, a collaboration of several national labs and universities. The Consortium, which is led by DOE’s Pacific Northwest National Laboratory, is striving to make batteries with an energy density of 500 watt-hours per kilogram—more than double the energy density of today’s state-of-the-art batteries.
This energy density cannot be achieved in the lithium-ion batteries powering most of today’s battery-operated devices—including phones, television remotes, and even electric vehicles. So, scientists needed to turn to lithium metal batteries to pursue their goals. These batteries possess a lithium metal anode, rather than the graphite anode present in lithium-ion batteries.
“The lithium metal battery is attractive because it can give twice the energy density of a battery with a graphite anode,” explained Rahman. “But there are lots of challenges to tackle.”
Brookhaven’s most recent research addresses one of these challenges—striking a balance between the charging speed and the cycle life.
The electrolyte that typically enables fast battery charging is also likely to be reactive with the lithium metal anode. If these chemical reactions proceed uncontrollably, the electrolyte decomposes and reduces the battery’s cycle life. To prevent this from happening, Brookhaven chemists set out to engineer the interphase.
Previous studies had indicated that the lithium metal anode could be stabilized with a cesium additive. But to increase the charging rate while maintaining the battery cycle life, the anode and cathode need to be stabilized simultaneously. The Brookhaven scientists believed cesium nitrate could serve this purpose for lithium metal batteries. As they had hypothesized, the positive cesium ion accumulated on the negatively charged lithium metal anode side of the battery, while the negative nitrate ion accumulated on the positively charged cathode.
To better understand how the cesium nitrate additive influenced the electrolyte composition and battery performance, the chemists brought the new batteries to the National Synchrotron Light Source II (NSLS-II), a DOE Office of Science user facility at Brookhaven Lab.
A gaze into the interphase
NSLS-II is one of the most advanced x-ray light sources in the world, producing light beams that are 10 billion times brighter than the sun. Of the 29 beamlines currently operating at NSLS-II, Rahman and Hu took advantage of the capabilities of four beamlines for their most recent research.
“NSLS-II is really a great facility for conducting battery research,” said Hu. “There is a breadth of techniques available, which enables us to conduct complete studies of complex materials.”
Among the four beamlines used by the chemists was the X-ray Powder Diffraction (XPD) beamline, a high energy diffraction beamline with photon beams that can contain more than three times the energy of conventional x-ray powder diffraction beamlines. For more than five years, Hu’s group has been leveraging these high energy beams for interphase studies that have led to a series of new understandings of battery chemistry.
The high-energy x-rays are capable of penetrating thick materials, like the anodes and cathodes within batteries. But they are also characterized by their high intensity, which enables the quick data collection necessary to take a “snapshot” of the elusive interphase.
“The XPD beamline is excellent because its x-rays have low absorption power and do not damage the interphase samples,” Hu elaborated. “One of the greatest challenges in characterizing interphase samples is their sensitivity to the x-ray beams, but we’ve characterized over 1,000 interphase samples at XPD without observing any damage to the samples.”
Some components of the interphase are crystalline, meaning that their atoms are neatly arranged. These components can typically be studied with conventional x-ray diffraction (XRD). But battery interphases also contain unorganized, amorphous components whose characterizations are beyond the capabilities of XRD. Instead, a technique called pair distribution function (PDF) analysis is needed. At the XPD beamline, led by Sanjit Ghose, scientists can conduct both techniques simultaneously. With these two techniques, the researchers can understand all the chemical species that evolve during the reactions that form the interphase components.
“We call this combined method total scattering,” explained Ghose, who is a co-author on the paper. “But these techniques are especially unique because they can characterize the structures of chemical species reliably—even if they are only present in trace amounts—which is needed for battery research.”
“Enyuan’s group is really becoming a champion of leveraging XPD’s total scattering techniques and its ability to not damage samples,” he added.
The scientists found that the cesium nitrate additive increased the presence of components known to make the interphase more protective. The XRD data, however, had a surprise in store. In addition to the typical crystalline components, a compound called cesium bis(fluorosulfonyl)imide was also identified.
“This component of the interphase had never been reported before,” said Rahman, emphasizing the novelty of the finding.
“But it’s not just about what we found,” added Hu. “It’s also what was missing from the interphase.”
Scientists studying batteries generally regard lithium fluoride as a necessary component of a good interphase. In fact, its presence and abundance are typically used to explain the impressive performance of lithium metal batteries. That’s why Rahman and Hu were especially surprised by its absence.
“We don’t know why it is not there,” Hu said. “But the fact that this lithium fluoride-free interphase enables a long cycle life and fast charging inspires us to revisit the current understanding of the interphase.”
Though the XPD beamline is adept at detecting trace amounts of interphase components, it is difficult to use the same x-ray beams to quantify these components—especially when some of them are present in such small amounts. So, the scientists brought their batteries to the Submicron Resolution X-ray Spectroscopy (SRX) beamline to quantitatively analyze how the different chemical elements collected on the battery electrodes and in their respective interphases after cycling.
To do this, the SRX beamline scientists used an ultra-sensitive technique called scanning x-ray fluorescence (XRF) microscopy. This technique, which is based on a known and calibrated standard, evaluates the chemical distribution of the interphase. The scanning XRF images confirmed that there was more cesium present in the anode interphase than the cathode interphase. With further scanning XRF analysis, the scientists revealed that the cesium nitrate additive prevented the breakdown of the transition metals that make up the cathode, contributing to the overall stabilization of the cathode and lithium metal battery.
The scientists also analyzed their samples at the Quick X-ray Absorption and Scattering (QAS) and the In situ and Operando Soft X-ray Spectroscopy (IOS) beamlines to verify that cesium accumulated on the lithium metal anode and nitrate accumulated on the cathode, respectively. Furthermore, the IOS beamline scientists confirmed that the cathode was stabilized with the cesium nitrate additive.
QAS beamline scientists take advantage of the beamline’s high energy x-rays, which can probe deep into the sample, to conduct hard x-ray absorption spectroscopy (XAS). Scientists at the IOS beamline, on the other hand, use low energy x-rays to directly probe atoms near the surface of the sample. Both techniques provide detailed analyses of the chemical and electronic states of the atoms present at the respective electrodes.
“Conducting complementary analyses at these additional beamlines helped us verify our design idea,” said Hu. The two XAS techniques were crucial for characterizing the anode and cathode as well as the interphase.
But the scientists’ analyses were not yet complete; they also had to check for stabilization of the lithium metal anode with the cesium nitrate additive. So, the scientists brought their batteries to the materials synthesis and characterization facility at the Center for Functional Nanomaterials (CFN), a DOE Office of Science user facility at Brookhaven Lab, to make use of the scanning electron microscope. The resulting microscope images showed that the lithium formed by electrochemical reactions deposits uniformly when the cesium nitrate is added to the electrolyte, thus contributing to the stabilization of the electrode and reinforcing the benefits of this additive.
"We really took advantage of all the resources available to us at Brookhaven Lab,” said Rahman.
By combining various techniques across two user facilities, the scientists were able to paint a full picture of how the lithium metal battery behaves with the cesium nitrate additive. This research contributes to a better understanding of interphase optimization and overall battery chemistry.
“Lithium metal batteries have come a long way, but they still have a long way to go. The interphase plays a key role in progress that still needs to be made,” Rahman said. “Our work has created new opportunities for interphase engineering, and I hope that this will inspire others to look at the interphase differently so that we can accelerate the development of lithium metal batteries.”
This work was supported by DOE’s Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office and DOE’s Office of Science. Operations at NSLS-II and CFN are supported by the Office of Science.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, Twitter, and Facebook.
JOURNAL
Nature Communications
ARTICLE TITLE
An inorganic-rich but LiF-free interphase for fast charging and long cycle life lithium metal batteries


