Maryland man receives first successful pig-to-human heart transplant
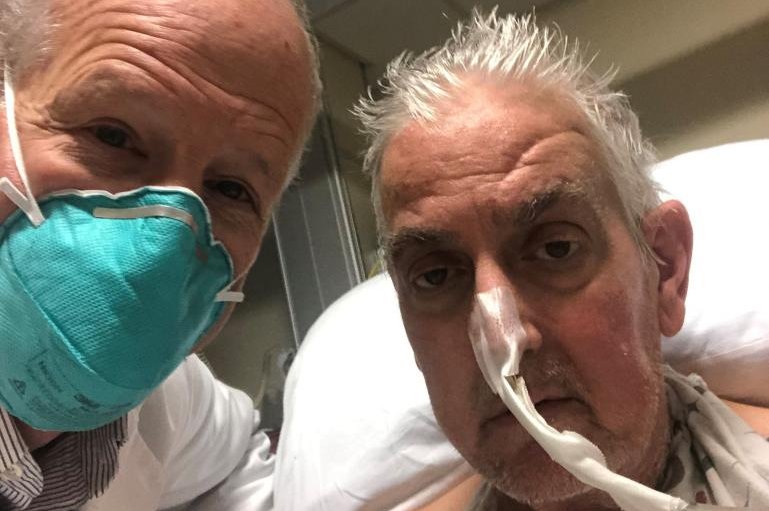
David Bennett, a 57-year-old from Maryland, became the first person to successfully receive a heart transplant from a genetically modified pig.

David Bennett, a 57-year-old from Maryland, became the first person to successfully receive a heart transplant from a genetically modified pig.
Photo courtesy University of Maryland Medical Center
Jan. 10 (UPI) -- A Maryland man became the first person to receive a successful pig-to-human heart transplant.
David Bennett, 57, is "still doing well" three days after the first-of-its-kind surgery, and will continue to be monitored to determine whether the transplant, conducted at the University of Maryland, provides lifesaving benefits, the university said in a statement.
"This was a breakthrough surgery and brings us one step closer to solving the organ shortage crisis. There are simply not enough donor hearts available to meet the long list of potential recipients," said Dr. Bartley Griffith, the doctor who performed the surgery.
"We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future."
RELATEDGene therapy may help cure sickle cell disease, study says
The surgery took nine hours and saw doctors replace Bennett's heart with one from a 1-year-old, 240-pound pig that was bred and had its genes edited specifically to provide organs to humans.
The pig had 10 genetic modifications that includes inactivating, or knocking out, four genes, including one that encodes a molecule that causes an aggressive human rejection response and a growth gene to prevent the pig's heart from continuing to grow after it was implanted.
Additionally, six human genes were inserted into the genome of the donor pigs to make the porcine organs more tolerable to the human immune system.
The transplant provided the first demonstration that a genetically modified animal heart can function like a human heart without being immediately rejected by the human body.
"It creates the pulse, it creates the pressure, it is his heart," Griffith told The New York Times. "It's working and it looks normal. We are thrilled, but we don't know what tomorrow will bring us. This has never been done before."
Bennett chose to undergo the experimental treatment, as he would have died without a new heart, had exhausted other methods of treatment and was not healthy enough to qualify for a heart from a human donor.
"It was either die or do this transplant," Bennett said, a day before the surgery. "I want to live. I know it's a shot in the dark, but it's my last choice."
The Food and Drug Administration granted emergency use authorization for the surgery on New Year's Eve through its expanded access provision.
Griffith said the procedure required some "clever plastic surgery to make everything fit" but added that once the clamp restricting blood supply to the organ was removed that "the heart fired right up" and "the animal heart began to squeeze."
Bennett remains connected to a heart-lung bypass machine, which he may be removed from as early as Tuesday, and is being closely monitored to ensure his body does not reject the new heart and for infections, including porcine retrovirus, a pig virus that can be transmitted to humans.
Jan. 10 (UPI) -- A Maryland man became the first person to receive a successful pig-to-human heart transplant.
David Bennett, 57, is "still doing well" three days after the first-of-its-kind surgery, and will continue to be monitored to determine whether the transplant, conducted at the University of Maryland, provides lifesaving benefits, the university said in a statement.
"This was a breakthrough surgery and brings us one step closer to solving the organ shortage crisis. There are simply not enough donor hearts available to meet the long list of potential recipients," said Dr. Bartley Griffith, the doctor who performed the surgery.
"We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future."
RELATEDGene therapy may help cure sickle cell disease, study says
The surgery took nine hours and saw doctors replace Bennett's heart with one from a 1-year-old, 240-pound pig that was bred and had its genes edited specifically to provide organs to humans.
The pig had 10 genetic modifications that includes inactivating, or knocking out, four genes, including one that encodes a molecule that causes an aggressive human rejection response and a growth gene to prevent the pig's heart from continuing to grow after it was implanted.
Additionally, six human genes were inserted into the genome of the donor pigs to make the porcine organs more tolerable to the human immune system.
The transplant provided the first demonstration that a genetically modified animal heart can function like a human heart without being immediately rejected by the human body.
"It creates the pulse, it creates the pressure, it is his heart," Griffith told The New York Times. "It's working and it looks normal. We are thrilled, but we don't know what tomorrow will bring us. This has never been done before."
Bennett chose to undergo the experimental treatment, as he would have died without a new heart, had exhausted other methods of treatment and was not healthy enough to qualify for a heart from a human donor.
"It was either die or do this transplant," Bennett said, a day before the surgery. "I want to live. I know it's a shot in the dark, but it's my last choice."
The Food and Drug Administration granted emergency use authorization for the surgery on New Year's Eve through its expanded access provision.
Griffith said the procedure required some "clever plastic surgery to make everything fit" but added that once the clamp restricting blood supply to the organ was removed that "the heart fired right up" and "the animal heart began to squeeze."
Bennett remains connected to a heart-lung bypass machine, which he may be removed from as early as Tuesday, and is being closely monitored to ensure his body does not reject the new heart and for infections, including porcine retrovirus, a pig virus that can be transmitted to humans.
The team behind the historic procedure say they hope the process can help solve the massive shortage of donor organs.
Surgeons in the United States have implanted the heart of a genetically modified pig into a human patient in a global first, the University of Maryland Medical School announced on Monday.
The procedure took place on Friday, and while the long-term prognosis for 57-year-old patient David Bennett is uncertain, the doctors hailed it as a "historic" milestone.
Bennett, who was still in the hospital recovering on Monday, said before the surgery: "It was either die or do this transplant. I want to live. I know it's a shot in the dark, but it's my last choice."
"I look forward to getting out of bed after I recover," he added, having been bedridden for months and hooked up to a heart-lung bypass machine.
Why was it necessary to use a pig's heart?
The US Food and Drug Administration issued an emergency authorization for the procedure on New Year's Eve, a final ray of hope for a patient who was not suitable for a traditional organ transplant.
"This was a breakthrough surgery and brings us one step closer to solving the organ shortage crisis," said Bartley Griffith, one of the surgeons on the team. "There are simply not enough donor human hearts available to meet the long list of potential recipients."
About 110,000 Americans are currently waiting for an organ transplant, and more than 6,000 patients die each year before getting one, according to official figures.
Muhammad Mohiuddin, who co-founded the university's cardiac xenotransplantation program, added the surgery was the culmination of years of research that involved transplanting pig organs into baboons.
The donor pigs are the result of gene editing that has removed certain markers that caused patients to reject the organs, or that led to the excessive growth sometimes seen in pigs.
Pig heart valves and pig skin grafts are already widely used on human patients.
Dr. Peter Chin-Hong, a doctor with the University of California, San Francisco, said the gene editing was the key difference between this attempt and earlier tries at the procedure.
"Our immune system is so vigrous" that it reacts to even "very minor perturbation that look foreign," and reject them he told DW, adding that the result also allowed patients to use fewer anti-rejection medications and "live a more nomal existence."
es/rt (AFP, Reuters)
In a medical first, doctors transplanted a pig heart into a patient


© Provided by The Canadian Press
In a medical first, doctors transplanted a pig heart into a patient in a last-ditch effort to save his life and a Maryland hospital said Monday that he's doing well three days after the highly experimental surgery.
While it’s too soon to know if the operation really will work, it marks a step in the decades-long quest to one day use animal organs for life-saving transplants. Doctors at the University of Maryland Medical Center say the transplant showed that a heart from a genetically modified animal can function in the human body without immediate rejection.
The patient, David Bennett, a 57-year-old Maryland handyman, knew there was no guarantee the experiment would work but he was dying, ineligible for a human heart transplant and had no other option, his son told The Associated Press.
“It was either die or do this transplant. I want to live. I know it’s a shot in the dark, but it’s my last choice,” Bennett said a day before the surgery, according to a statement provided by the University of Maryland School of Medicine.
On Monday, Bennett was breathing on his own while still connected to a heart-lung machine to help his new heart. The next few weeks will be critical as Bennett recovers from the surgery and doctors carefully monitor how his heart is faring.
There’s a huge shortage of human organs donated for transplant, driving scientists to try to figure out how to use animal organs instead. Last year, there were just over 3,800 heart transplants in the U.S., a record number, according to the United Network for Organ Sharing, which oversees the nation’s transplant system.
"If this works, there will be an endless supply of these organs for patients who are suffering,” said Dr. Muhammad Mohiuddin, scientific director of the Maryland university's animal-to-human transplant program.
But prior attempts at such transplants — or xenotransplantation — have failed, largely because patients’ bodies rapidly rejected the animal organ. Notably, in 1984, Baby Fae, a dying infant, lived 21 days with a baboon heart.
The difference this time: The Maryland surgeons used a heart from a pig that had undergone gene-editing to remove a sugar in its cells that’s responsible for that hyper-fast organ rejection. Several biotech companies are developing pig organs for human transplant; the one used for Friday's operation came from Revivicor, a subsidiary of United Therapeutics.
“I think you can characterize it as a watershed event,” Dr. David Klassen, UNOS’ chief medical officer, said of the Maryland transplant.
Still, Klassen cautioned that it’s only a first tentative step into exploring whether this time around, xenotransplantation might finally work.
The Food and Drug Administration, which oversees such experiments, allowed the surgery under what’s called a “compassionate use” emergency authorization, available when a patient with a life-threatening condition has no other options.
It will be crucial to share the data gathered from this transplant before extending it to more patients, said Karen Maschke, a research scholar at the Hastings Center, who is helping develop ethics and policy recommendations for the first clinical trials under a grant from the National Institutes of Health.
“Rushing into animal-to-human transplants without this information would not be advisable,” Maschke said.
Over the years, scientists have turned from primates to pigs, tinkering with their genes.
Just last September, researchers in New York performed an experiment suggesting these kinds of pigs might offer promise for animal-to-human transplants. Doctors temporarily attached a pig’s kidney to a deceased human body and watched it begin to work.
The Maryland transplant takes their experiment to the next level, said Dr. Robert Montgomery, who led that work at NYU Langone Health.
“This is a truly remarkable breakthrough," he said in a statement. "As a heart transplant recipient, myself with a genetic heart disorder, I am thrilled by this news and the hope it gives to my family and other patients who will eventually be saved by this breakthrough.”
The surgery last Friday took seven hours at the Baltimore hospital. Dr. Bartley Griffith, who performed the surgery, said the patient’s condition — heart failure and an irregular heartbeat — made him ineligible for a human heart transplant or a heart pump.
Griffith had transplanted pig hearts into about 50 baboons over five years, before offering the option to Bennett.
“We’re learning a lot every day with this gentleman,” Griffith said. “And so far, we’re happy with our decision to move forward. And he is as well: Big smile on his face today.”
Pig heart valves also have been used successfully for decades in humans, and Bennett’s son said his father had received one about a decade ago.
As for the heart transplant, “He realizes the magnitude of what was done and he really realizes the importance of it,” David Bennett Jr. said. “He could not live, or he could last a day, or he could last a couple of days. I mean, we’re in the unknown at this point.”
__
AP Medical Writer Lauran Neergaard contributed.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
Carla K. Johnson, The Associated Press
In a medical first, doctors transplanted a pig heart into a patient in a last-ditch effort to save his life and a Maryland hospital said Monday that he's doing well three days after the highly experimental surgery.
While it’s too soon to know if the operation really will work, it marks a step in the decades-long quest to one day use animal organs for life-saving transplants. Doctors at the University of Maryland Medical Center say the transplant showed that a heart from a genetically modified animal can function in the human body without immediate rejection.
The patient, David Bennett, a 57-year-old Maryland handyman, knew there was no guarantee the experiment would work but he was dying, ineligible for a human heart transplant and had no other option, his son told The Associated Press.
“It was either die or do this transplant. I want to live. I know it’s a shot in the dark, but it’s my last choice,” Bennett said a day before the surgery, according to a statement provided by the University of Maryland School of Medicine.
On Monday, Bennett was breathing on his own while still connected to a heart-lung machine to help his new heart. The next few weeks will be critical as Bennett recovers from the surgery and doctors carefully monitor how his heart is faring.
There’s a huge shortage of human organs donated for transplant, driving scientists to try to figure out how to use animal organs instead. Last year, there were just over 3,800 heart transplants in the U.S., a record number, according to the United Network for Organ Sharing, which oversees the nation’s transplant system.
"If this works, there will be an endless supply of these organs for patients who are suffering,” said Dr. Muhammad Mohiuddin, scientific director of the Maryland university's animal-to-human transplant program.
But prior attempts at such transplants — or xenotransplantation — have failed, largely because patients’ bodies rapidly rejected the animal organ. Notably, in 1984, Baby Fae, a dying infant, lived 21 days with a baboon heart.
The difference this time: The Maryland surgeons used a heart from a pig that had undergone gene-editing to remove a sugar in its cells that’s responsible for that hyper-fast organ rejection. Several biotech companies are developing pig organs for human transplant; the one used for Friday's operation came from Revivicor, a subsidiary of United Therapeutics.
“I think you can characterize it as a watershed event,” Dr. David Klassen, UNOS’ chief medical officer, said of the Maryland transplant.
Still, Klassen cautioned that it’s only a first tentative step into exploring whether this time around, xenotransplantation might finally work.
The Food and Drug Administration, which oversees such experiments, allowed the surgery under what’s called a “compassionate use” emergency authorization, available when a patient with a life-threatening condition has no other options.
It will be crucial to share the data gathered from this transplant before extending it to more patients, said Karen Maschke, a research scholar at the Hastings Center, who is helping develop ethics and policy recommendations for the first clinical trials under a grant from the National Institutes of Health.
“Rushing into animal-to-human transplants without this information would not be advisable,” Maschke said.
Over the years, scientists have turned from primates to pigs, tinkering with their genes.
Just last September, researchers in New York performed an experiment suggesting these kinds of pigs might offer promise for animal-to-human transplants. Doctors temporarily attached a pig’s kidney to a deceased human body and watched it begin to work.
The Maryland transplant takes their experiment to the next level, said Dr. Robert Montgomery, who led that work at NYU Langone Health.
“This is a truly remarkable breakthrough," he said in a statement. "As a heart transplant recipient, myself with a genetic heart disorder, I am thrilled by this news and the hope it gives to my family and other patients who will eventually be saved by this breakthrough.”
The surgery last Friday took seven hours at the Baltimore hospital. Dr. Bartley Griffith, who performed the surgery, said the patient’s condition — heart failure and an irregular heartbeat — made him ineligible for a human heart transplant or a heart pump.
Griffith had transplanted pig hearts into about 50 baboons over five years, before offering the option to Bennett.
“We’re learning a lot every day with this gentleman,” Griffith said. “And so far, we’re happy with our decision to move forward. And he is as well: Big smile on his face today.”
Pig heart valves also have been used successfully for decades in humans, and Bennett’s son said his father had received one about a decade ago.
As for the heart transplant, “He realizes the magnitude of what was done and he really realizes the importance of it,” David Bennett Jr. said. “He could not live, or he could last a day, or he could last a couple of days. I mean, we’re in the unknown at this point.”
__
AP Medical Writer Lauran Neergaard contributed.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
Carla K. Johnson, The Associated Press
No comments:
Post a Comment