Unraveling the complex role of climate in dengue dynamics
Institute for Basic Science
image:
Existing methods used to analyze the impact of climate variables on dengue fever showed that small changes in the data resulted in varying relationships between climatic factors and dengue incidence. In contrast, the recently developed GOBI method (Park et al., Nature Communications, 2023) consistently provides accurate results.
view moreCredit: Institute for Basic Science
The research team led by KIM Jae Kyoung, Professor in the Department of Mathematical Sciences at KAIST and Chief Investigator of the Biomedical Mathematics Group at the Institute for Basic Science (IBS), has unveiled new insights into how weather influences the spread of dengue fever. Their study identifies temperature and rainfall as critical factors driving the global surge in dengue cases and offers actionable strategies for mitigating the disease's impact.
Dengue fever, a mosquito-borne disease, poses an increasingly alarming public health challenge. According to the World Health Organization, reported dengue cases surged from 4.1 million in 2023 to over 10.6 million in 2024 in North and South America alone. This number represents the highest global count ever reported. While climatic factors such as temperature and rainfall are known to drive this trend, their complex relationship with dengue dynamics remains poorly understood. Previous studies have struggled to reconcile conflicting findings - some suggest rainfall accelerates dengue transmission, while others indicate it suppresses it.
The IBS research team hypothesized that these inconsistencies stem from the limitations of traditional methods that focus on linear relationships or independent effects. To address this, the researchers utilized GOBI (General ODE-Based Inference), a novel causal inference framework that the IBS group developed in 2023. This method captures both nonlinear and combined effects of climatic factors, enabling a more nuanced analysis of the relationship between weather and dengue incidence.
The study focused on 16 regions of the Philippines, selected for their diverse climatic conditions, to examine how temperature and rainfall jointly affect dengue dynamics. There were distinct patterns of dengue regulation across the Philippines, driven by the combined effects of temperature and rainfall. Rising temperatures were consistently associated with higher dengue incidence across all regions. On the other hand, rainfall showed contrasting effects depending on the location of the region. In eastern areas, rainfall increased dengue incidence, while in western regions, rainfall suppressed it.
The most important factor turned out to be the variation in dry season length, which was identified as critical to explaining the contrasting effects of rainfall. In regions with low variation in dry season length, rainfall tended to flush out stagnant water, reducing mosquito breeding sites and suppressing dengue transmission. On the other hand, in regions with high variation in dry season length, sporadic rainfall created new breeding sites and weakened the flushing effect, driving an increase in mosquito populations and dengue cases.
The role of dry season length has largely been overlooked in previous research but proved to be a decisive factor in this study. This discovery offers a fresh perspective on the intricate relationship between rainfall and dengue dynamics.
To validate their findings, the researchers extended their analysis to Puerto Rico, a region with distinct climatic zones. Data from municipalities including San Juan, Adjuntas, and Ponce exhibited similar regulation patterns, underscoring the generalizability of the results.
“Our findings provide robust evidence for how climatic factors influence dengue transmission in diverse environments. This represents a significant step toward understanding how climate change may impact mosquito-borne diseases globally,” said first author Olive R. CAWIDING.
The study’s findings have immediate applications for optimizing dengue intervention strategies. For low-variation regions, natural flushing effects during the rainy season may allow for scaled-back intervention efforts, freeing resources for other priorities. Specifically, consistent and year-round intervention efforts are necessary in high-variation regions to counteract the breeding-friendly conditions created by sporadic rainfall.
Furthermore, the study highlights the importance of monitoring dry season length as a predictive factor for dengue outbreaks. By tailoring strategies to specific regional climate patterns, public health agencies can allocate resources more efficiently and effectively to combat the spread of dengue.
The study represents a significant turning point in understanding how climate change impacts not only dengue fever, but also other climate-driven diseases such as malaria, influenza, and the Zika virus.
CI KIM Jae Kyoung stated, “This research is crucial as it overcomes the limitations of traditional methods for detecting nonlinear relationships and clearly elucidates the complex interactions between climatic variables and infectious diseases through an advanced causal inference algorithm. This approach can also be applied to the analysis of various diseases linked to climate.”
While the study provides robust insights, the researchers acknowledge certain limitations, including the lack of mosquito population data and socioeconomic factors such as healthcare accessibility and human mobility. Future studies with access to more granular data, such as weekly dengue incidence and mosquito dynamics, could further refine these findings.
The study, titled “Disentangling climate’s dual role in dengue dynamics: a multi-region causal analysis study,” was published online in Science Advances, a sister journal of Science.
Figure 2. Analysis of five years of climate change and dengue fever incidence data from 16 regions in the Philippines reveals clear patterns in the relationships between climatic variables and dengue incidence 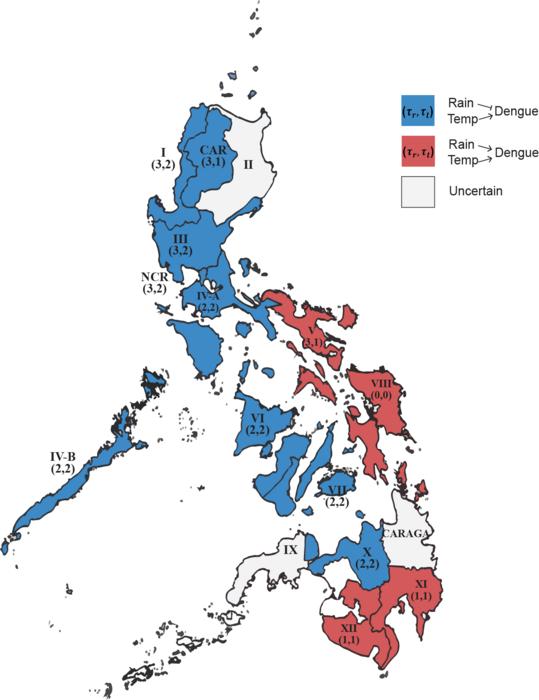
Temperature consistently acts as a key factor driving increased dengue fever incidence across all regions. In contrast, rainfall had location-dependent effects: it increased dengue incidence in the eastern regions and reduced incidence in the western regions.
In regions where the dry season length is consistent, rainfall tends to suppress dengue fever incidence, whereas in regions with inconsistent dry season duration, rainfall tends to promote dengue fever incidence.
(From left to right) Olive R. CAWIDING (IBS/KAIST, first author), CI KIM Jae Kyoung (IBS/KAIST, corresponding author).
Credit
Institute for Basic Science
Journal
Science Advances
Method of Research
Data/statistical analysis
Subject of Research
Not applicable
Article Title
Disentangling climate’s dual role in dengue dynamics: a multi-region causal analysis study
Article Publication Date
12-Feb-2025
Re-emergence of dengue serotype 3 may increase severity of outbreaks of the disease in Brazil
The population is not immunized against DENV-3. Meanwhile, DENV-1 and -2 continue to circulate, experts warn
Fundação de Amparo à Pesquisa do Estado de São Paulo
image:
Brazil is the country most affected by dengue in the Americas
view moreCredit: Marcelo Camargo/Agência Brasil
The reemergence of dengue virus serotype 3 (DENV-3) in Brazil after 17 years could help worsen fresh outbreaks of the disease there since the population is not immunized against this serotype, and serotypes 1 and 2 (DENV-1 and DENV-2) continue to circulate.
The warning features in an article written by researchers at the São José do Rio Preto Medical School (FAMERP) in São Paulo state, and published in the Journal of Clinical Virology.
“The last significant DENV-3 epidemic in Brazil, and more specifically in São José do Rio Preto, occurred more than 15 years ago [in 2007]. DENV-1 and DENV-2 are continuously circulating in Brazil. If DENV-3 establishes itself again and the situation persists [with these serotypes co-circulating], severe forms of dengue epidemic could result. This is precisely the situation we’re experiencing right now in São José do Rio Preto,” Maurício Lacerda Nogueira, a professor at FAMERP and last author of the article, told Agência FAPESP.
For 20 years, Nogueira and colleagues have conducted a Thematic Project that is supported by FAPESP and includes genomic and epidemiological surveillance of dengue and other diseases caused by insect-born viruses (arboviruses) in São José do Rio Preto, where dengue has been endemic for several decades, with a succession of outbreaks caused by different serotypes.
“The annual average temperature in São José do Rio Preto is about 25 °C , and rainfall averages about 2,000 millimeters per year. This warm wet weather creates ideal conditions for the mosquitos that transmit arboviruses to breed and makes the city a suitable place for genomic and epidemiological monitoring of arboviruses, including dengue. We’ve been doing this for a long time, so we’re able to make reliable epidemiological inferences,” Lacerda said.
The researchers have documented a rise in cases of the disease caused by DENV-3 in the city since late 2023 via active surveillance of arboviruses in patients with dengue-like symptoms treated at Hospital de Base and UPAs (public clinics specializing in medium-complexity cases).
Thirty-one blood samples collected between November 2023 and November 2024 tested positive for DENV-3. The most common symptoms were muscle pain, headache, and fever.
“In 2023-24, we had a dengue epidemic in São José do Rio Preto, caused mainly by DENV-1 and -2. In mid-2024, DENV-1 almost disappeared, and DENV-2 became the main agent. The number of DENV-3 cases then began to rise, and it’s now the main agent in the city,” Lacerda said.
Studies conducted by other groups show that the last outbreak of dengue in Brazil, in 2021, was caused by DENV-1, and that sequential infection by DENV-3 is associated with increased severity during epidemics.
“However, we didn’t observe increased severity in the patients who participated in our study,” Lacerda said.
Need for active surveillance
The researchers also sequenced the genomes and analyzed the phylogenies of the viral isolates collected from the blood samples donated by patients with acute fever. The results of the analysis showed that the DENV-3 strain in question belonged to the same lineage as the one identified in Florida (USA) and the Caribbean, and differed from the strains of DENV-3 that circulated in Brazil in the 2000s.
These findings suggest that the outbreak of DENV-3 in Florida and the Caribbean in 2022-24 probably contributed to the introduction of the virus to Brazil and its spread throughout the country, according to the researchers.
“This demonstrates the extent to which molecular and genomic surveillance of circulating DENV strains is crucial to public health preparedness efforts and the response to the surge in cases of the disease,” Lacerda said.
Dengue transmission is widespread across tropical and subtropical regions worldwide. However, at-risk areas have expanded in past decades, owing mainly to climate change and an increased range of Aedes aegypti, the mosquito that transmits the virus, the article notes.
Brazil is the most affected country in the Americas and has long been hyperendemic for all dengue virus serotypes. In recent years, DENV-1 and -2 have been the most common serotypes in circulation. Although DENV-3 was detected during the period discussed in the article, the number of cases was very small. In fact, fewer than 100 cases were reported nationwide between 2010 and 2022. However, the number of cases reported increased in 2023, reaching 106, and jumped to 1,008 in 2024.
“We’ve been studying dengue in Brazil since 2010, and the epidemiological pattern is similar to that observed for SARS-CoV-2 during the COVID-19 pandemic. When a different serotype emerges, it escapes the population’s established immunity, and an epidemic occurs shortly afterward. We’re seeing this now with DENV-3,” Lacerda said.
About FAPESP
The São Paulo Research Foundation (FAPESP) is a public institution with the mission of supporting scientific research in all fields of knowledge by awarding scholarships, fellowships and grants to investigators linked with higher education and research institutions in the state of São Paulo, Brazil. FAPESP is aware that the very best research can only be done by working with the best researchers internationally. Therefore, it has established partnerships with funding agencies, higher education, private companies, and research organizations in other countries known for the quality of their research and has been encouraging scientists funded by its grants to further develop their international collaboration.
Journal
Journal of Clinical Virology
Article Title
Early insights of dengue virus serotype 3 (DENV-3) re-emergence in São Paulo, Brazil
A single-dose breakthrough: PfSPZ-LARC vaccines offer transformative protection against malaria
Scientists at Sanaria and Seattle Children’s Research Institute’s Center for Global Infectious Disease Research (CGIDR) have unveiled a groundbreaking malaria vaccine, Sanaria® PfSPZ-LARC2 Vaccine, designed to provide high-level protection with just one dose. This innovative approach leverages decades of research and cutting-edge genetic engineering to combat one of the world’s deadliest diseases.
Malaria continues to impose a devastating burden worldwide, with 263 million cases and nearly 600,000 deaths reported in 2023, most of those being in children under 5. The World Health Organization (WHO) has called for an ambitious goal of 90% protection against Plasmodium falciparum (Pf) infection. Despite significant investment and the introduction of vaccines such as RTS,S and R21, that goal has not been attained. Recent data suggest that PfSPZ-LARC2’s single-dose approach could redefine malaria prevention and elimination efforts globally and have put the WHO goal within sight.
The Science Behind the PfSPZ-LARC2 Vaccine
LARC (Late liver stage-Arresting and Replication-Competent) describes genetically weakened parasites that replicate in the liver, but halt progression before reaching the blood stage, ensuring the recipient of the vaccine remains safe, symptom-free from malaria, and protected from disease. Using advanced genetic engineering, researchers deleted two critical parasite genes, Mei2 and LINUP, from the Pf genome. These deletions ensure that the parasites replicate in the liver but cannot advance to the blood stage, rendering the vaccine incapable of causing disease or transmitting, while preserving protection.
This innovative dual-gene deletion approach builds on the team’s earlier research with PfSPZ-LARC1, in which only the Mei2 gene was deleted. By adding the LINUP deletion, the team further increased the vaccine’s safety, making LARC2 a promising candidate for widespread use. Preclinical studies have shown that LARC vaccines are significantly more potent and protective than existing options.
Promising Results and Future Trials
A pivotal study published in Nature Medicine in January 2025 showcased the remarkable potential of LARC vaccines. Researchers at Leiden University Medical Center tested a LARC1 parasite with the deletion of the Mei2 gene alone (called GA2 by the Leiden team) and demonstrated that this provided 90% protection against controlled human malaria infection (CHMI) after a single immunization via mosquito bite—an unprecedented result in malaria vaccine research.
The success of GA2 strongly validates the use of genetically weakened parasites to achieve high levels of immunity. While GA2 demonstrated exceptional efficacy with a single-gene deletion (Mei2), the PfSPZ-LARC2 Vaccine builds upon this foundation by incorporating dual deletions to ensure that PfSPZ-LARC2 preserves the protective efficacy observed in GA2 while meeting rigorous safety standards necessary for widespread use.
Although GA2 demonstrated exceptional efficacy, its administration method (via mosquito bites) cannot be used for a deployed vaccine. In contrast, the injectable PfSPZ-LARC2 Vaccine meets regulatory standards for clinical development and can be manufactured and distributed on a large scale.
Clinical trials of PfSPZ-LARC2 are scheduled for 2025 in the U.S., Germany, and Burkina Faso. These trials will evaluate the vaccine’s safety and efficacy in diverse populations and environments. Results from these trials are expected to provide critical insights into the vaccine’s global deployment potential within the next three years.
Expert Commentary
The breakthrough has garnered enthusiastic support from leading experts:
“We are excited about assessing PfSPZ-LARC2 Vaccine in Burkina Faso, as it is the only malaria vaccine in development that has the potential of achieving the WHO goal of at least 90% protection against Pf infection,” said Professor Sodiomon Sirima of GRAS (Groupe de Recherche Action en Santé) and principal investigator on the upcoming Burkina Faso trial.
“We have worked for two decades to develop a highly protective, cost-effective PfSPZ vaccine. PfSPZ vaccines have provided the highest levels of protection against controlled human malaria infection and the only malaria vaccines shown to protect against Pf infection for two years without boosting, including in pregnancy. PfSPZ-LARC2 Vaccine is our 3rd generation vaccine, and expected to be our flagship going forward,” said Dr. Stephen L. Hoffman, Sanaria’s CEO.
In a News & Views article in Nature Medicine, Dr. Stefan Kappe of CGIDR pointed out that immunization with GA2 gave unprecedented protection against malaria infection with a single shot, indicating that LARC vaccines are potentially transformational tools that could make malaria eradication with a vaccine a reality.
Addressing the Global Malaria Crisis
Despite over $4 billion in annual investments for malaria control measures, global case numbers and deaths have remained stagnant over the past decade. The WHO’s call for a vaccine that provides at least 90% protection underscores the urgent need for innovative solutions. The development of PfSPZ-LARC2 Vaccine to prevent infection, clinical disease, and transmission is timely as it comes at a time of increasing Pf drug resistance, enhancing spread of malaria due to climate change, and uncertainty of government commitments to global health, all which have the potential to lead to rapid increases in malaria cases and deaths.
PfSPZ-LARC2 Vaccine’s single-dose regimen, unparalleled protection rates, and potential for broad accessibility position it as a game-changer in global health efforts. By targeting the parasite at a critical stage of its life cycle, this vaccine could finally make malaria elimination a reality.
About Sanaria Inc.
Founded in 2003, Sanaria Inc. is dedicated to developing and commercializing whole-parasite malaria vaccines that provide high-level, long-lasting protection against Plasmodium falciparum, the primary cause of severe malaria worldwide. Sanaria’s research and development operations are headquartered in Rockville, Maryland. Learn more at sanaria.com.
Forward-Looking Statements
This news release contains certain forward-looking statements that involve known and unknown risks and uncertainties, which may cause actual results to differ materially from anticipated results or achievements expressed or implied by the statements made. Such statements include the availability of an effective vaccine, the expectations for eliminating malaria, and beliefs concerning the suitability of a successful vaccine. These forward-looking statements are further qualified by important factors that could cause actual results to differ materially from those in the forward-looking statements. These factors include, without limitation, the Company’s ability to raise sufficient funds, the regulatory approval process, clinical trials results, the Company’s patent portfolio, dependence on key personnel and other risks associated with vaccine development. For further information contact Alexander Hoffman, sanaria@sanaria.com, 301-770-3222.
No comments:
Post a Comment