Nature’s Alchemy: Cellular Waste Transformed Into Essential Chemicals
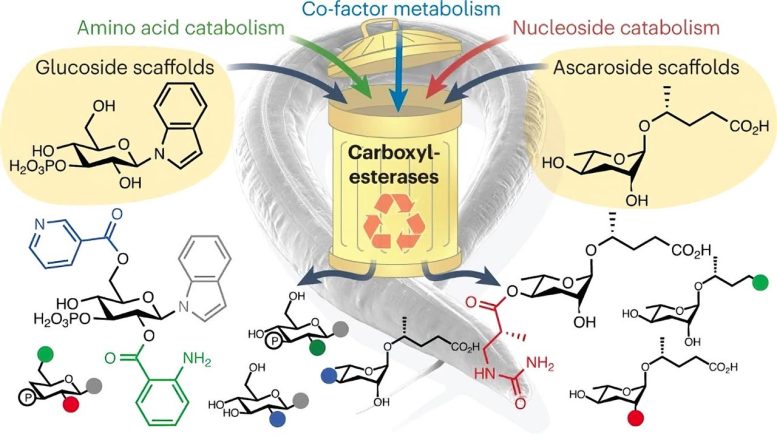
In a recent Nature Chemical Biology article, researchers revealed a previously unknown biochemical recycling process in animals that uses cellular waste to produce new, vital chemicals. These chemicals play important roles in regulating behavior, development, and aging. Contrary to previous belief, genes thought to code for carboxylesterases, enzymes that hydrolyze esters, actually assemble a variety of new metabolites from cellular waste. This discovery could revolutionize our understanding of animal and human functioning, opening up new research avenues to explore the structure and function of over 100,000 distinct, uninvestigated chemicals. Credit: Boyce Thompson Institute
A recent study uncovers a biochemical recycling process in animals that uses cellular waste to create vital chemicals, revolutionizing our understanding of animal and human functioning. This discovery opens up new research avenues for exploring over 100,000 uninvestigated chemicals.
A new perspective published in the journal Nature Chemical Biology uncovers a previously unknown biochemical recycling process in animals. The authors review a flurry of recent papers demonstrating that animals extensively recycle biochemical waste to produce novel chemicals that play key roles in biology, from regulating behavior to development and aging.
These studies show that the genes previously thought to code for carboxylesterases, enzymes that hydrolyze esters, actually play a pivotal role in assembling a wide range of new metabolites from building blocks generally considered “cellular waste.” Surprisingly, the so-called carboxylesterases were found to contribute to the formation of esters and amide bonds, a function opposite to that predicted by computational algorithms.
“This discovery reveals that our understanding of biochemistry remains largely incomplete,” says the perspective’s lead author, Frank Schroeder, a professor at Boyce Thompson Institute (BTI). “This research has the potential to revolutionize our understanding of how animals, including humans, function.”
Recent investigations indicate that animals and humans may produce over 100,000 distinct chemicals, most of which have not been investigated. This unknown structure space is a treasure trove of chemicals, which may hold the key to understanding many biological processes. One major challenge to understanding how these metabolites contribute to survival is that the enzymes that produce them are also unknown.
“The discovery of this biochemical recycling mechanism opens up exciting new avenues for future research, with the potential to dramatically accelerate the structural and functional annotation of unknown metabolites,” says Chester Wrobel, a graduate student in the Schroeder lab and co-author of the perspective.
Reference: “Repurposing degradation pathways for modular metabolite biosynthesis in nematodes” by Chester J. J. Wrobel and Frank C. Schroeder, 6 April 2023, Nature Chemical Biology.
DOI: 10.1038/s41589-023-01301-w
No comments:
Post a Comment