MINING.COM Staff Writer | August 9, 2021 |

Pouring mercury from small tube into pan which contains flour gold mixed with the natural dirt. (Reference image from Library of Congress, CC0).
Researchers at ETH university in Zürich have produced nanocrystals made of two different metals using an amalgamation process whereby a liquid metal penetrates a solid one.

In a paper published in the journal Science Advances, the group led by Maksym Yarema and Vanessa Wood explained that nanocrystals are nanometre-sized spheres consisting of regularly arranged atoms. When they are semiconductors, they are used in new generation TV screens. On the other hand, intermetallic nanocrystals, in which two different metals combine to form a crystal lattice, have made a name for themselves as they promise improved applications in areas such as catalysis, data storage, and medicine.
But up until now, it has only been possible to make nanocrystals out of a few metal pairings of the thousands of possible combinations.
The new technique, however, allows one to realize nearly all possible combinations of intermetallic nanocrystals.
In conventional procedures for producing nanocrystals made of a single metal, the metal atoms are introduced in molecular form, for instance as salts, into a solution in which the nanocrystals then form.
According to the scientists, theoretically, that can also be done with two different metals, but in practice, it is difficult to combine distinctly dissimilar metals in the reactor. This is why Yarema and the group resorted to amalgamation.
The process, used for centuries in gold mining, consists of adding liquid mercury to dissolve other metals. However, amalgamation also works with any other liquid metals.
Using this approach at the nanoscale means that the reaction starts with the dispersion of nanocrystals containing a single metal, for instance, silver. Then, the atoms of the second metal— which could also be gallium as it melts at 30 degrees Celsius—are added in molecular form as amides, a compound of carbon, hydrogen, and nitrogen, while the mixture is heated to around 300 degrees.
Initially, the high temperature causes the chemical bonds in the gallium-amide to break up, allowing liquid gallium to accumulate on the silver nanocrystals. The actual amalgamation process begins, during which liquid gallium creeps into the solid silver. Over time a new crystal lattice is formed, in which eventually silver and gallium atoms are regularly arranged. Then everything is cooled down again, and after 10 minutes the nanocrystals are ready.
“We are amazed how efficient the amalgamation is at the nanoscale. Having one liquid metal component is the key to fast and uniform alloying within each nanocrystal,” Yarema said in a media statement.
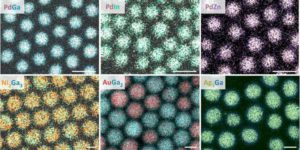
Using the same technique, the researchers have already produced different intermetallic nanocrystals such as gold-gallium, copper-gallium, and palladium-zinc. They say that the amalgamation process itself can be precisely steered.
Through the number of secondary atoms, introduced into the solution as amides, the proportion of the metals in the nanocrystals can be accurately controlled.
“Because the amalgamation synthesis of nanocrystals enables so many new compositions, we cannot wait to see them at work in improved catalysis, plasmonics, or lithium-ion batteries,” Yarema said.