With more than 2.5 million people now infected worldwide in the COVID-19 pandemic, Germany has authorized the first clinical trial of a coronavirus vaccine. The first human tests will begin before the end of April.
German Health Minister Jens Spahn has announced the first clinical trials of a coronavirus vaccine. The Paul Ehrlich Institute (PEI), the regulatory authority which helps develop and authorizes vaccines in Germany, has given the go-ahead for the first clinical trial of BNT162b1, a vaccine against the SARS-CoV-2 virus.
It was developed by cancer researcher and immunologist Ugur Sahin and his team at pharmaceutical company BioNTech, and is based on their prior research into cancer immunology. Sahin previously taught at the University of Mainz before becoming the CEO of BioNTech.
In a joint conference call on Wednesday with researchers from the Paul Ehrlich Institute, Sahin said BNT162b1 constitutes a so-called RNA vaccine. He explained that innocuous genetic information of the SARS-CoV-2 virus is transferred into human cells with the help of lipid nanoparticles, a non-viral gene delivery system. The cells then transform this genetic information into a protein, which should stimulate the body's immune reaction to the novel coronavrius.
Watch video Global race to find coronavirus vaccine
Numerous vaccines in development
Aside from BNT162b1, which is now in the stage 1 testing phase, BioNTech — jointly with Pfizer — is working on three other similar mRNA vaccines. PEI head Klaus Cichutek, meanwhile, has said other pharmaceutical companies are also developing vaccines against SARS-CoV-2, based on a variety of vaccine platforms in Europe, China and the United States.
Read more: Pandemic driving tech solutions in sub-Saharan Africa
The first medical tests of BNT162b1 will involve 200 healthy volunteers between the ages of 18 and 55. The aim is to determine the immune response and whether the vaccine causes any unwanted side effects.
"Trials of vaccine candidates in humans are an important milestone on the road to safe and efficacious vaccines against COVID-19 for the population in Germany and internationally," the PEI said in a statement.
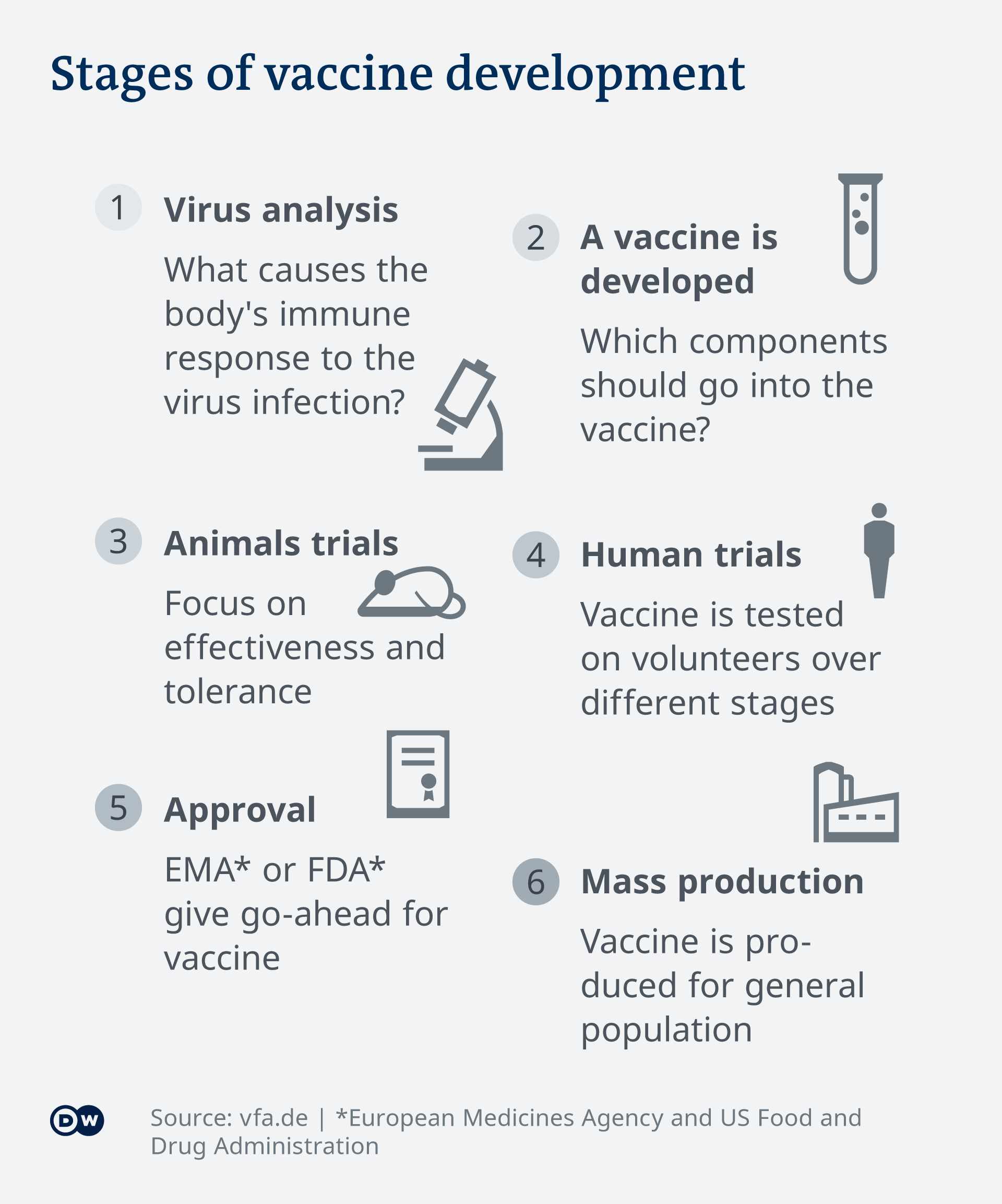
Cichutek said testing would be completed by June, at the earliest. After this stage is complete, the PEI will determine if the vaccine can progress to further trial stages. Cichutek warned, however, that an approved vaccine was unlikely to be ready for the general public in 2020.
More than 2.5 million people have been infected by the COVID-19 pandemic in the last four months, and at least 179,000 people have died.
RESEARCHERS AND THEIR SELF-EXPERIMENTS
An oral vaccination against coronavirus
Courage, curiosity or complete hubris? It's probably a mixture of all these things that causes many scientists to test their own inventions on themselves first. According to the Global Times, a Chinese doctor not only developed an oral vaccine against the SARS-CoV-2 but also tried it out himself. So far, he hasn't seen any side effects.
MORE PHOTO STORIES HERE 12345678
DW RECOMMENDS
Coronavirus: Doctors in Italy cry foul over protection
Did authorities in Lombardy get it badly wrong? There are allegations that mismanagement at retirement homes caused hundreds of deaths and that authorities failed to provide adequate guidelines for hospitals and doctors. (21.04.2020)
Fighting coronavirus isolation over the phone
People living in isolation because of the coronavirus pandemic can become extremely lonely. That is why people like Ellen Ladnorg volunteer to talk to them for several hours a week on the telephone. (21.04.2020)
Date 22.04.2020
DW RECOMMENDS
Coronavirus: Doctors in Italy cry foul over protection
Did authorities in Lombardy get it badly wrong? There are allegations that mismanagement at retirement homes caused hundreds of deaths and that authorities failed to provide adequate guidelines for hospitals and doctors. (21.04.2020)
Fighting coronavirus isolation over the phone
People living in isolation because of the coronavirus pandemic can become extremely lonely. That is why people like Ellen Ladnorg volunteer to talk to them for several hours a week on the telephone. (21.04.2020)
Date 22.04.2020
No comments:
Post a Comment