Staff Writer | August 12, 2022

Cement. (Reference image by Pong Koedpoln, Pxhere).
Researchers at the University of Delaware are exploring ways to use clay-like topsoil materials from the moon or Mars as the basis for extraterrestrial cement, which would be used for buildings, housing, or rocket landing pads.

“If we’re going to live and work on another planet like Mars or the moon, we need to make concrete. But we can’t take bags of concrete with us—we need to use local resources,” Norman Wagner, co-author of the study that explores this idea, said in a media statement.
According to Wagner, succeeding in the creation of ET cement will require a binder to glue the starting materials together through chemistry. One requirement for this construction material is that it must be durable enough for the vertical launch pads needed to protect man-made rockets from swirling rocks, dust and other debris during liftoff or landing. Most conventional construction materials, such as ordinary cement, are not suitable under space conditions.
To overcome these limitations, the UD scientists decided to convert simulated lunar and Martian soils into geopolymer cement, which is considered a good substitute for conventional cement. The research team also created a framework to compare different types of geopolymer cement and their characteristics.
In their paper published in Advances in Space Research, the researchers explain that geopolymers are inorganic polymers formed from aluminosilicate minerals found in common clays everywhere from Newark, Delaware’s White Clay Creek, to Africa. When mixed with a solvent that has a high pH, such as sodium silicate, the clay can be dissolved, freeing the aluminum and silicon inside to react with other materials and form new structures—like cement. Soils on the moon and Mars contain common clays, too.
Simulating moon and Martian soils
These similarities made co-authors Maria Katzarova and Jennifer Mills wonder if it was possible to activate simulated moon and Martian soils to become concrete-like building materials using geopolymer chemistry. Thus, they systematically prepared geopolymer binders from a variety of known simulated soils in the same exact way and compared the materials’ performance, which hadn’t been done before.
The researchers mixed various simulated soils with sodium silicate then cast the geopolymer mixture into ice-cube-like moulds and waited for the reaction to occur.
After seven days, they measured each cube’s size and weight, then crushed it to understand how the material behaves under load. Specifically, they wanted to know if slight differences in chemistry between simulated soils affected the material’s strength.
“When a rocket takes off there’s a lot of weight pushing down on the landing pad and the concrete needs to hold, so the material’s compressive strength becomes an important metric,” Wagner said. “At least on earth, we were able to make materials in little cubes that had the compressive strength necessary to do the job.”
The researchers calculated how much terrestrial material astronauts would need to take with them to build a landing pad on the surface of the moon or Mars. Turns out, the estimated amount is well within the payload range of a rocket, anywhere from hundreds to thousands of kilograms.
Performance under different conditions
The team also subjected the samples to different environments present in space, including vacuum and low and high temperatures.
Under vacuum, some of the material samples did form cement, while others were only partially successful. However, overall, the geopolymer cement’s compressive strength decreased under vacuum, compared to geopolymer cubes cured at room temperature and pressure. This raises new considerations, depending on the material’s purpose.
“There’s going to be a tradeoff between whether we need to cast these materials in a pressurized environment to ensure the reaction forms the strongest material or whether we can get away with forming them under vacuum, the normal environment on the moon or Mars, and achieve something that’s good enough,” Mills said.
Meanwhile, under low temperatures of about -80 degrees Celsius, the geopolymer materials didn’t react at all.
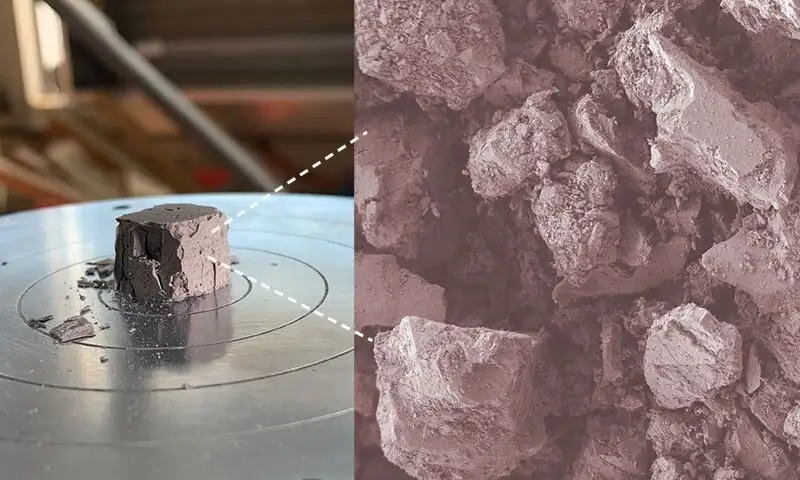
A crushed geopolymer cube made from simulated lunar topsoil, inset shows magnification of lunar topsoil particles which have been activated and reacted to form the geopolymer binder. (Image courtesy of the University of Delaware).
At high temperatures, about 600 degrees Celsius, the researchers found that every moon-like sample got stronger.
This was not surprising, given how the kinetics were hindered at low temperatures. The research team also saw changes in the physical nature of the geopolymer cement under heat.
“The geopolymer bricks became much more brittle when we heated them up, shattering as opposed to becoming compressed or breaking in two,” Mills pointed out. “That could be important if the material is going to be subjected to any type of external pressure.”
Based on their results, the researchers said that chemical composition and particle size may play an important role in material strength. For example, smaller particles increase the available surface area, making them easier to react and potentially leading to greater overall material strength. Another possible factor: the amount of aluminosilicate content in the starting materials, which can be tricky to estimate when added solutions may also contain small concentrations of these materials and contribute to material performance.
The group notes that understanding what affects material strength is important, too, since astronauts will be sourcing our topsoil materials from different places on planets—and maybe even different planets altogether.
These results also can be used on earth to make geopolymer cement that is better for the environment and can be sourced from a wider variety of local materials. Geopolymer cement requires less water than is needed to make traditional cement because the water itself is not consumed in the reaction. Instead, the water can be recovered and reused, a plus in water-limited environments from arid earthly landscapes to outer space.
At high temperatures, about 600 degrees Celsius, the researchers found that every moon-like sample got stronger.
This was not surprising, given how the kinetics were hindered at low temperatures. The research team also saw changes in the physical nature of the geopolymer cement under heat.
“The geopolymer bricks became much more brittle when we heated them up, shattering as opposed to becoming compressed or breaking in two,” Mills pointed out. “That could be important if the material is going to be subjected to any type of external pressure.”
Based on their results, the researchers said that chemical composition and particle size may play an important role in material strength. For example, smaller particles increase the available surface area, making them easier to react and potentially leading to greater overall material strength. Another possible factor: the amount of aluminosilicate content in the starting materials, which can be tricky to estimate when added solutions may also contain small concentrations of these materials and contribute to material performance.
The group notes that understanding what affects material strength is important, too, since astronauts will be sourcing our topsoil materials from different places on planets—and maybe even different planets altogether.
These results also can be used on earth to make geopolymer cement that is better for the environment and can be sourced from a wider variety of local materials. Geopolymer cement requires less water than is needed to make traditional cement because the water itself is not consumed in the reaction. Instead, the water can be recovered and reused, a plus in water-limited environments from arid earthly landscapes to outer space.
No comments:
Post a Comment