by University of Sheffield
Credit: ACS
Sustainable Chemistry & Engineering (2022). DOI: 10.1021/acssuschemeng.2c06218
A new paper, published in the ACS Sustainable Chemistry & Engineering journal, found that pine needles could be used to produce renewable fuels and value-added chemicals, such as preservatives used in agriculture, using only water as a solvent.
Between 6 and 8 million real Christmas trees are sold every year in the UK, with an estimated seven million making their way to landfill at the end of the festive period.
Not only is this costly, but once in landfill, each tree will release 16 kg of greenhouse gases as they decompose, producing methane gas, which is 25 times more potent than carbon dioxide (CO2).
Earlier University research from 2018 found that useful products could be made from the chemicals extracted from pine needles when processed. With the aid of heat and solvents, the 2018 study established that the chemical structure of pine needles could be broken down into a liquid product (bio-oil), which could be used in the production of sweeteners, paint, adhesives and vinegar and a solid by-product (bio-char), which could be used in other industrial chemical processes.
The research in this new paper built on this previous University research project. It was carried out by María Andérez-Fernández, a University of Valladolid Ph.D. student, who was visiting the University of Sheffield's Department of Chemical and Biological Engineering at the time much of the research was conducted, under the supervision of Dr. James McGregor.
Dr. McGregor, Senior Lecturer in the Department of Chemical and Biological Engineering, said, "One of the things that we do when reacting carbon dioxide to capture CO2 is to use a metal to promote the reaction. This can be inefficient and expensive, so we went back to some of the work we've done previously with pine needles, because we realized that we could potentially use these to promote turning the carbon dioxide into formic acid."
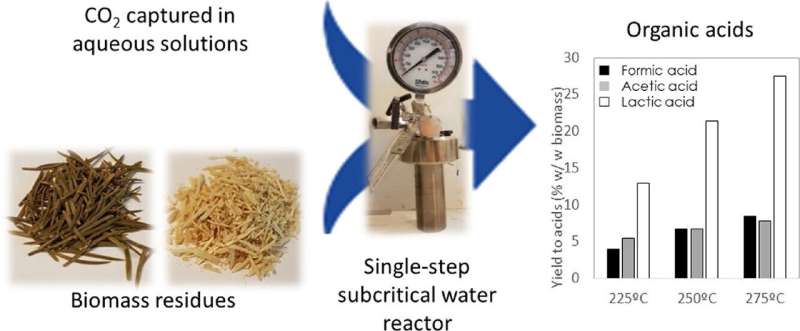
This published work demonstrates how reacting carbon dioxide and water together at high temperatures can make useful products.
Maria Andérez-Fernández said, "We found that instead of the metal and the carbon dioxide reacting, we could react carbon dioxide with pine needles and water at high temperatures and a fraction of the pine needles would turn into the same product as the CO2.
"Carbon dioxide is introduced as sodium bicarbonate, commonly known as baking soda or bicarbonate of soda. This co-conversion with captured carbon dioxide, that we didn't have before in the previous pine needles research, has found that the two things enhance the conversion of each other, making it more efficient and in this case, making more of the end product—formic acid."
Formic acid has many applications, it can be used in fuel cells to store and transport hydrogen, which can then be used as a power source, offering a clean alternative to fossil fuels, as it doesn't have any harmful emissions.
It is also widely used as a preservative for foods and an antibacterial agent in livestock feed, as well as in the manufacture of leather and rubber.
Maria added, "With these results, this study sets a new strategy for CO2 and residual biomass valorization (the process of reusing waste materials and converting them into more useful products) to produce renewable fuels and value-added chemicals, using only water as a solvent and producing a simultaneous reaction that simplifies the process and makes it more efficient."
The study looked at using different waste materials—sugar cane as well as pine needles— and concluded although sugar cane worked more effectively, refineries could use mixed biomass feedstock at different times of the year when different waste products are more abundant, for example, using pine needles in January.
Dr. McGregor added, "This could create a challenge in the process design, as currently refineries use feedstock that is constant throughout the year. So, if what you are feeding in as your reactants change throughout the year, this would have to be built in. But it's a place we would like to get to so that in January, we are using the millions of pine needles readily available rather than them going to landfill."
If pine needles were collected after Christmas and processed in this way, the chemicals could be used to replace less sustainable chemicals currently used in industry.
This could lead to a decrease in the UK's carbon footprint by reducing the use of artificial plastic-based Christmas trees, reducing the amount of biomass waste going to landfill and potentially saving an estimated 100,000 metric tons of greenhouse gases, currently released every year by landfilled Christmas trees.
More information: María Andérez-Fernández et al, Synergistic Hydrothermal Conversion of Aqueous Solutions of CO2 and Biomass Waste Liquefaction into Formate, ACS Sustainable Chemistry & Engineering (2022). DOI: 10.1021/acssuschemeng.2c06218
Provided by University of Sheffield
Explore further
A new paper, published in the ACS Sustainable Chemistry & Engineering journal, found that pine needles could be used to produce renewable fuels and value-added chemicals, such as preservatives used in agriculture, using only water as a solvent.
Between 6 and 8 million real Christmas trees are sold every year in the UK, with an estimated seven million making their way to landfill at the end of the festive period.
Not only is this costly, but once in landfill, each tree will release 16 kg of greenhouse gases as they decompose, producing methane gas, which is 25 times more potent than carbon dioxide (CO2).
Earlier University research from 2018 found that useful products could be made from the chemicals extracted from pine needles when processed. With the aid of heat and solvents, the 2018 study established that the chemical structure of pine needles could be broken down into a liquid product (bio-oil), which could be used in the production of sweeteners, paint, adhesives and vinegar and a solid by-product (bio-char), which could be used in other industrial chemical processes.
The research in this new paper built on this previous University research project. It was carried out by María Andérez-Fernández, a University of Valladolid Ph.D. student, who was visiting the University of Sheffield's Department of Chemical and Biological Engineering at the time much of the research was conducted, under the supervision of Dr. James McGregor.
Dr. McGregor, Senior Lecturer in the Department of Chemical and Biological Engineering, said, "One of the things that we do when reacting carbon dioxide to capture CO2 is to use a metal to promote the reaction. This can be inefficient and expensive, so we went back to some of the work we've done previously with pine needles, because we realized that we could potentially use these to promote turning the carbon dioxide into formic acid."
This published work demonstrates how reacting carbon dioxide and water together at high temperatures can make useful products.
Maria Andérez-Fernández said, "We found that instead of the metal and the carbon dioxide reacting, we could react carbon dioxide with pine needles and water at high temperatures and a fraction of the pine needles would turn into the same product as the CO2.
"Carbon dioxide is introduced as sodium bicarbonate, commonly known as baking soda or bicarbonate of soda. This co-conversion with captured carbon dioxide, that we didn't have before in the previous pine needles research, has found that the two things enhance the conversion of each other, making it more efficient and in this case, making more of the end product—formic acid."
Formic acid has many applications, it can be used in fuel cells to store and transport hydrogen, which can then be used as a power source, offering a clean alternative to fossil fuels, as it doesn't have any harmful emissions.
It is also widely used as a preservative for foods and an antibacterial agent in livestock feed, as well as in the manufacture of leather and rubber.
Maria added, "With these results, this study sets a new strategy for CO2 and residual biomass valorization (the process of reusing waste materials and converting them into more useful products) to produce renewable fuels and value-added chemicals, using only water as a solvent and producing a simultaneous reaction that simplifies the process and makes it more efficient."
The study looked at using different waste materials—sugar cane as well as pine needles— and concluded although sugar cane worked more effectively, refineries could use mixed biomass feedstock at different times of the year when different waste products are more abundant, for example, using pine needles in January.
Dr. McGregor added, "This could create a challenge in the process design, as currently refineries use feedstock that is constant throughout the year. So, if what you are feeding in as your reactants change throughout the year, this would have to be built in. But it's a place we would like to get to so that in January, we are using the millions of pine needles readily available rather than them going to landfill."
If pine needles were collected after Christmas and processed in this way, the chemicals could be used to replace less sustainable chemicals currently used in industry.
This could lead to a decrease in the UK's carbon footprint by reducing the use of artificial plastic-based Christmas trees, reducing the amount of biomass waste going to landfill and potentially saving an estimated 100,000 metric tons of greenhouse gases, currently released every year by landfilled Christmas trees.
More information: María Andérez-Fernández et al, Synergistic Hydrothermal Conversion of Aqueous Solutions of CO2 and Biomass Waste Liquefaction into Formate, ACS Sustainable Chemistry & Engineering (2022). DOI: 10.1021/acssuschemeng.2c06218
Provided by University of Sheffield
Explore further
No comments:
Post a Comment