Scientists use tardigrade proteins for human health breakthrough

University of Wyoming researchers' study of how microscopic creatures called tardigrades survive extreme conditions has led to a major breakthrough that could eventually make life-saving treatments available to people where refrigeration isn't possible.
Thomas Boothby, an assistant professor of molecular biology, and colleagues have shown that natural and engineered versions of tardigrade proteins can be used to stabilize an important pharmaceutical used to treat people with hemophilia and other conditions without the need for refrigeration—even amid high temperatures and other difficult conditions. The findings are detailed in Scientific Reports.
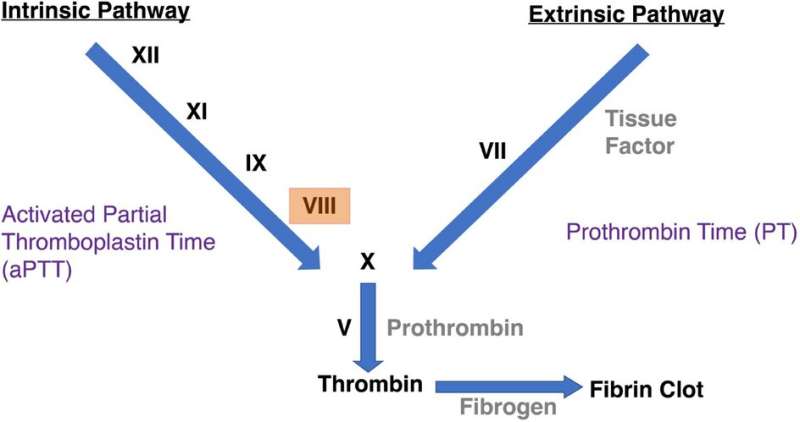
The pharmaceutical known as human blood clotting Factor VIII is an essential therapeutic used to treat genetic disease and instances of extreme bleeding. Despite being critical and effective in treating patients in these circumstances, Factor VIII has a serious shortcoming, in that it is inherently unstable. Without stabilization within a precise temperature range, Factor VIII will break down.
"In underdeveloped regions, during natural disasters, during space flight or on the battlefield, access to refrigerators and freezers, as well as ample electricity to run this infrastructure, can be in short supply. This often means that people who need access to Factor VIII do not get it," Boothby says. "Our work provides a proof of principle that we can stabilize Factor VIII, and likely many other pharmaceuticals, in a stable, dry state at room or even elevated temperatures using proteins from tardigrades—and, thus, provide critical lifesaving medicine to everyone everywhere."
Measuring less than half a millimeter long, tardigrades—also known as water bears—can survive being completely dried out; being frozen to just above absolute zero (about minus 458 degrees Fahrenheit, when all molecular motion stops); heated to more than 300 degrees Fahrenheit; irradiated several thousand times beyond what a human could withstand; and even survive the vacuum of outer space. They are able to do so, in part, by manufacturing a sugar called trehalose and a protein called CAHS D.
According to the research paper, Boothby and his colleagues fine-tuned the biophysical properties of both trehalose and CAHS D to stabilize Factor VIII, noting that CAHS D is most suitable for the treatment. The stabilization allows Factor VIII to be available in austere conditions without refrigeration, including repeated dehydration/rehydration, extreme heat and long-term dry storage.
The researchers believe the same thing can be done with other biologics—pharmaceuticals containing or derived from living organisms—such as vaccines, antibodies, stem cells, blood and blood products.
"This study shows that dry preservation methods can be effective in protecting biologics, offering a convenient, logistically simple and economically viable means of stabilizing life-saving medicines," Boothby says. "This will be beneficial not only for global health initiatives in remote or developing parts of the world, but also for fostering a safe and productive space economy, which will be reliant on new technologies that break our dependence on refrigeration for the storage of medicine, food and other biomolecules."
Boothby and other researchers hope that their discoveries can be applied to address other societal and global health issues as well, including water scarcity. For example, their work might lead to better ways of generating engineered crops that can cope with harsh environments.
More information: Maxwell H. Packebush et al, Natural and engineered mediators of desiccation tolerance stabilize Human Blood Clotting Factor VIII in a dry state, Scientific Reports (2023). DOI: 10.1038/s41598-023-31586-9. www.nature.com/articles/s41598-023-31586-9
Provided by University of Wyoming
Researchers reveal new knowledge of microscopic creature's durability
The world's toughest animal could one day help save your life
By Bronwyn Thompson
March 20, 2023
Water bear, moss piglet, scientific marvel: the tiny tardigrade
DepositphotosThey’ve been fired from a gas gun to test their candidacy for panspermia, are believed to have survived the Beresheet lunar probe's crash-landing on the Moon, can live without water, withstand radiation, survive being frozen and are expected to be one of the final forms of life on Earth when the sun begins to dim in about five billion years.
So it’s no surprise that everyone’s favorite microscopic critter has yet another superpower up its chubby sleeves: some clever chemistry unique to the tardigrade that can stabilize medicines without refrigeration. It has huge potential for getting life-saving treatment to those who need it.
Researchers at the University of Wyoming have homed in on one of the tardigrade’s key survival skills, anhydrobiosis. The team believed that the animal’s ability to enter reversible suspended animation when faced with extreme water loss from cells, could provide the same stable dry storage for biologic medicines that would otherwise require the chilled environment.
Biologics – vaccines, antibodies, stem cells, blood and other blood products – are derived from living organisms and require cold conditions to prevent heat breaking down the protein and destroying it. One that relies on this prohibitive cold-chain infrastructure is human blood-clotting (coagulation) factor VIII (FVIII), which among its therapeutic applications are treating genetic diseases such as hemophilia A and those with extreme physical trauma and bleeding.
By harnessing a specific protein and sugar that the microscopic water bear produces in anhydrobiosis, the researchers found that it could offer FVIII similar desiccation shields, meaning the biologic could be dehydrated and then rehydrated for use without the loss of its natural qualities. What's more, their study shows the FVIII remained stable for 10 weeks in its treated form.
“In underdeveloped regions, during natural disasters, during space flight or on the battlefield, access to refrigerators and freezers, as well as ample electricity to run this infrastructure, can be in short supply,” said Thomas Boothby, assistant professor of molecular biology at UW. “Our work provides a proof of principle that we can stabilize factor VIII, and likely many other pharmaceuticals, in a stable, dry state at room or even elevated temperatures using proteins from tardigrades – and, thus, provide critical live-saving medicine to everyone, everywhere.”
Using the Hypsibius dujardini species, the team fine-tuned a treatment based on the cytosolic abundant heat soluble (CAHS) proteins and the sugar trehalose. In particular, the CAHS D protein protects enzymes in its dehydrated state, forming gel-like filaments to keep the animal’s cell structure intact. When hydration returns, the filaments retreat without causing cellular stress.
Taking the biophysical properties of CAHS D and trehalose, the team was able to stabilize the FVIII, opening the door to develop this transport and storage technology across the spectrum of biologics.
“This study shows that dry preservation methods can be effective in protecting biologics, offering a convenient, logistically simple and economically viable means of stabilizing life-saving medicines,” said Boothby. “This will be beneficial not only for global health initiatives in remote or developing parts of the world, but also for fostering a safe and productive space economy, which will be reliant on new technologies that break our dependence on refrigeration for the storage of medicine, food and other biomolecules.”
The study was published in the journal Scientific Reports.
Source: University of Wyoming
Tardigrade protein can keep medicines stabilized without refrigeration
The results demonstrated that FVIII could remain stable in its treated form for up to ten weeks.
Mrigakshi Dixit
Created: Mar 21, 2023/2023/03/21/image/jpeg/TpoZKDCTLJNRNMRmu3JRnbGxRFxLD543hkzk2sRQ.jpg)
Tardigrade stock image.
The tiny tardigrades, which measure less than half a millimeter in length, are frequently referred to as scientific marvels.
They can withstand harsh conditions such as freezing cold or super hot temperatures; they can survive without water, thrive in outer space, and can combat harmful radiation. These critters do, in fact, have a survival superpower.
It turns out scientists can tap into their superpower, which could help save medicines in unsuitable conditions and places.
Understanding tardigrade's survival skill
Scientists have delved into understanding how tardigrades survive frigid conditions, which could pave the way for medicines to be stabilized without refrigeration. This study is led by scientists from the University of Wyoming.
These microscopic creatures, also known as water bears, survive by producing trehalose (sugar) and CAHS D (protein). They do so through a process called anhydrobiosis — meaning life without water in Greek.
For this, the team fine-tuned the biophysical properties of CAHS D and trehalose in order to stabilize Factor VIII. Factor VIII is a critical protein in human blood clotting.
The results demonstrated that FVIII could remain stable in its treated form for up to ten weeks. It survived in the absence of refrigeration, dehydration/rehydration, dry storage, and even heat.
“Our work provides a proof of principle that we can stabilize Factor VIII, and likely many other pharmaceuticals, in a stable, dry state at room or even elevated temperatures using proteins from tardigrades — and, thus, provide critical life-saving medicine to everyone everywhere,” said Thomas Boothby, assistant professor of molecular biology, in a press release
Medicines require cold storage
Biologics, which include vaccines, antibodies, stem cells, blood, and other blood products, require cold temperatures to prevent heat from breaking down and destroying the protein.
Among these is factor VIII (FVIII), which has a significant pharmaceutical application and is heavily reliant on cold-chain infrastructure. It is used to treat genetic diseases such as hemophilia A and individuals who have experienced physical trauma and bleeding.
“In underdeveloped regions, during natural disasters, during space flight or on the battlefield, access to refrigerators and freezers, as well as ample electricity to run this infrastructure, can be in short supply. This often means that people who need access to Factor VIII do not get it,” said Boothby.
This method is also considered "logistically simple and economically viable" for preserving medicines. The details have been published in the journal Scientific Reports.
Study abstract:
Biologics, pharmaceuticals containing or derived from living organisms, such as vaccines, antibodies, stem cells, blood, and blood products are a cornerstone of modern medicine. However, nearly all biologics have a major deficiency: they are inherently unstable, requiring storage under constant cold conditions. The so-called ‘cold-chain’, while effective, represents a serious economic and logistical hurdle for deploying biologics in remote, underdeveloped, or austere settings where access to cold-chain infrastructure ranging from refrigerators and freezers to stable electricity is limited. To address this issue, we explore the possibility of using anhydrobiosis, the ability of organisms such as tardigrades to enter a reversible state of suspended animation brought on by extreme drying, as a jumping off point in the development of dry storage technology that would allow biologics to be kept in a desiccated state under not only ambient but elevated temperatures. Here we examine the ability of different protein and sugar-based mediators of anhydrobiosis derived from tardigrades and other anhydrobiotic organisms to stabilize Human Blood Clotting Factor VIII under repeated dehydration/rehydration cycles, thermal stress, and long-term dry storage conditions. We find that while both protein and sugar-based protectants can stabilize the biologic pharmaceutical Human Blood Clotting Factor VIII under all these conditions, protein-based mediators offer more accessible avenues for engineering and thus tuning of protective function.
No comments:
Post a Comment