Promoting nitric oxide electroreduction to ammonia over electron-rich Cu modulated by Ru doping
As an important nitrogen-containing chemical, ammonia plays a vital role in the production of fertilizers, explosives and fine chemicals. At present, ammonia has been mainly manufactured from H2 and N2 under high temperature (300-500 oC) and high pressure (200-300 atm). This process consumes huge amounts of energy and discharges vast amounts of greenhouse gas. Thus, electrochemical ammonia synthesis (EAS) has attracted intensive attention.
Presently, EAS mainly focuses on the electrochemical reduction of N2. However, the inherent inertness of N2 severely limits the Faradaic efficiency and yield rate of ammonia. Simultaneously, massive nitric oxide (NO) is discharged and causes serious environmental concerns. The present commercial treatment technologies aim to convert NO into environmental-friendly but useless N2.
From the view of "turning waste into wealth", developing novel EAS strategies by adopting NO as a nitrogen source is a win-win opportunity. But, the development of this technology is retarded by the lack of efficient electrocatalysts. Moreover, identifying intermediates and unveiling the reaction mechanism of NO electroreduction reaction (NOER) is critical for the design and construction of advanced electrocatalysts.
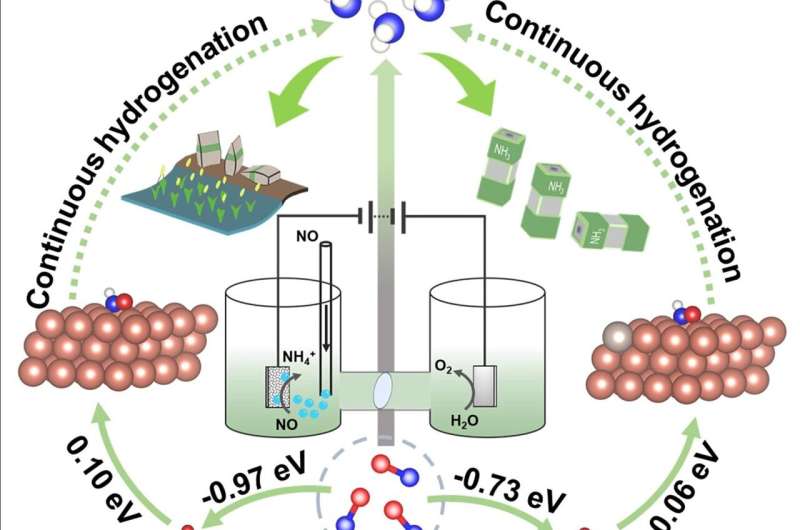
Recently, Prof. Bin Zhang and colleagues in Tianjin University constructed a series of Ru-doped Cu materials through in situ electroreduction of the corresponding metal hydroxides. The optimized Ru0.05Cu0.95exhibited superior electrocatalytic performance for ammonia synthesis by using NO/Ar (1/4, n/n) as the feedstocks (Faradaic efficiency: 64.9%, Yield rate: 17.68 µmol cm-2 h-1), obviously outperforming Cu counterpart (Faradaic efficiency: 33.0%, Yield rate: 5.73 µmol cm-2 h-1). The alternating-N pathway of NOER over Ru0.05Cu0.95 was confirmed based on the detected intermediates from electrochemical in situ Fourier transform infrared (FTIR) spectroscopy and online differential electrochemical mass spectrometry (DEMS).
Experimental and theoretical simulations unveiled that the decreased d-band center of surface Cu caused by Ru doping reduced the reaction energy of the rate-limiting hydrogenation step and the desorption energy of NH3, inducing the improvement of NOER performance. This work may open a new avenue for rational design and construction of efficient electrocatalysts for NO-to-NH3 conversion.
No comments:
Post a Comment