CRIMINAL CAPITALI$M; BIG PHARMA
Poisonous cough syrup killed 300 children across the world – officials fear more could soon dieSamaan Lateef
Wed, March 8, 2023

Safitri Puspa Rani cries as she holds a picture of her late son Panghegar Bhumi in Jakarta. The boy died from fatal acute kidney injury days after a doctor prescribed him cough syrup linked to more than 200 child deaths in Indonesia
- BAY ISMOYO/AFP via Getty Images
International investigators probing the fatal poisoning of 300 young children with deadly cough syrup fear the lethal ingredient may still be circulating in global supply chains.
There is frustration that authorities in India, where two separate batches of the syrups came from, are putting the country’s commercial interests above patient safety by withholding vital information.
It is understood that the World Health Organisation’s (WHO) Substandard and Falsified Medical Products team has yet to receive any data or documents collected by Indian officials investigating the production of cough syrup at Marion Biotech – one of two Indian pharmaceuticals embroiled in the scandal.
This is despite products made by the company being linked to the deaths of 20 children in Uzbekistan and the WHO appealing to the Indian authorities for information.
“We would have expected and hoped for a much quicker response from the Indian authorities,” said a source close to the WHO investigation.
Getting information about the manufacturing companies involved is vital so it can be understood whether or not there are tainted or mislabeled raw ingredients circulating in international supply chains, which could be used by other manufacturers unwittingly.
“This is the nightmare scenario,” said the source. “If we don’t get to the bottom of this, it could pop up again later.”
International investigators probing the fatal poisoning of 300 young children with deadly cough syrup fear the lethal ingredient may still be circulating in global supply chains.
There is frustration that authorities in India, where two separate batches of the syrups came from, are putting the country’s commercial interests above patient safety by withholding vital information.
It is understood that the World Health Organisation’s (WHO) Substandard and Falsified Medical Products team has yet to receive any data or documents collected by Indian officials investigating the production of cough syrup at Marion Biotech – one of two Indian pharmaceuticals embroiled in the scandal.
This is despite products made by the company being linked to the deaths of 20 children in Uzbekistan and the WHO appealing to the Indian authorities for information.
“We would have expected and hoped for a much quicker response from the Indian authorities,” said a source close to the WHO investigation.
Getting information about the manufacturing companies involved is vital so it can be understood whether or not there are tainted or mislabeled raw ingredients circulating in international supply chains, which could be used by other manufacturers unwittingly.
“This is the nightmare scenario,” said the source. “If we don’t get to the bottom of this, it could pop up again later.”

A photograph shows collected cough syrups in Banjul, The Gambia
- MILAN BERCKMANS/AFP via Getty Images
The cough syrup deaths, which occurred in Uzbekistan, the Gambia and Indonesia, have become the biggest tainted medicine scandal since contaminated cough syrup killed 365 people in Panama in 2006.
The latest disaster has seen hundreds of small children – most of them under five – die of kidney failure after being given over-the-counter cough syrups by their parents.
In each case, there has been confirmed or suspected contamination of the medicines with solvents called diethylene glycol (DEG) or ethylene glycol (EG).
These chemicals can be toxic in even small amounts, initially causing abdominal pain, headaches, vomiting, diarrhoea and acute kidney damage that often proves fatal.
Penny Ward, a visiting professor in pharmaceutical medicine at King’s College London, said initial gastrointestinal symptoms “can be overlooked or put down to other causes in countries where such problems are often seen in children.”
“Hence these kids then present in the later phases when renal failure has occurred and … [by then] it may be too late,” she added.
In the UK and other developed countries, it is rare for children to die in these circumstances, not only because drug regulation is stronger, but because dialysis and transplantation are available.
But in those nations where such services are hard to access, such as the Gambia or Uzbekistan, poisoning of this type can be terribly drawn out, eventually culminating in a painful death.
The cough syrup deaths, which occurred in Uzbekistan, the Gambia and Indonesia, have become the biggest tainted medicine scandal since contaminated cough syrup killed 365 people in Panama in 2006.
The latest disaster has seen hundreds of small children – most of them under five – die of kidney failure after being given over-the-counter cough syrups by their parents.
In each case, there has been confirmed or suspected contamination of the medicines with solvents called diethylene glycol (DEG) or ethylene glycol (EG).
These chemicals can be toxic in even small amounts, initially causing abdominal pain, headaches, vomiting, diarrhoea and acute kidney damage that often proves fatal.
Penny Ward, a visiting professor in pharmaceutical medicine at King’s College London, said initial gastrointestinal symptoms “can be overlooked or put down to other causes in countries where such problems are often seen in children.”
“Hence these kids then present in the later phases when renal failure has occurred and … [by then] it may be too late,” she added.
In the UK and other developed countries, it is rare for children to die in these circumstances, not only because drug regulation is stronger, but because dialysis and transplantation are available.
But in those nations where such services are hard to access, such as the Gambia or Uzbekistan, poisoning of this type can be terribly drawn out, eventually culminating in a painful death.
‘He fell into a coma and died’
Ebrima Sagnia, from Old Yundum in Gambia, west Africa, witnessed his two-year-old son Lamin die over five heart wrenching days in September last year.
He had given the little boy syrup purchased at a local pharmacy because he was feeling out of sorts.
But after being given the medicine, Lamin’s condition worsened and he didn’t appear to be passing urine. He was taken to hospital but doctors found his kidneys had failed.
“They said they suspect the cause of the kidney failure is due to the paracetamol syrup that we gave him,” Mr Sagnia told the Telegraph. “My child eventually died in the hospital after being admitted for five days.”
Gambia’s parliament estimates at least 70 children died in the country of cough syrup poisoning between June 4 and November 6 last year.
An inquiry concluded “all the cases of acute kidney injury are linked to the consumption of the contaminated medical products imported” from India.
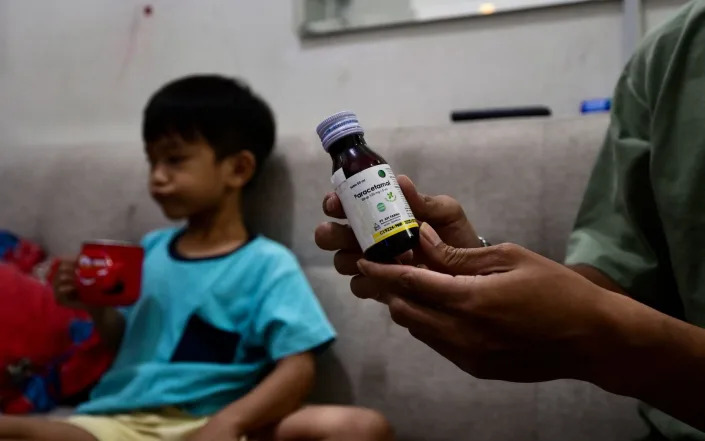
Riski Agri holds a bottle of cough syrup consumed by his Farrazka at their house in Jakarta. The five-year-old underwent dialysis for his failing kidneys after he took the medicine - BAY ISMOYO/AFP via Getty Images
Five thousand miles away in the Central Asian nation of Uzbekistan, another 20 children died in frighteningly similar circumstances.
Jasur Lafasov’s 18-month-old son, Ilhom Khayrullaev, passed away just after Christmas.
The toddler had developed a nasty cough and the local doctor prescribed cough syrup. Initially the cough improved but he then fell ill and stopped passing urine.
“On December 28, after work I went to see my son and his condition improved a little”, said Mr Lafasov. “He looked at me when I called his name. After that, he fell into a coma and died on the same day.”
Tests showed his kidneys and liver had both failed. Doctors at the hospital in Karshi said he had been poisoned by the cough syrup – again imported from India.
The third country to record deaths is Indonesia, where as many as 200 children have died.
Tainted cough syrups have also recently turned up in Senegal, Philippines, and East Timor, according to the WHO, although no deaths have yet been reported.
Spotlight on ‘the world’s pharmacy’
It is not known, but investigators suspect that some or all of the poisoning cases may be connected.
Respectable pharmaceutical manufacturers rigorously test the raw materials they purchase for toxins and they also test samples of their finished medicines.
This should be enforced by regulation in the country of manufacture but it is evident that it does not always happen.
“The international system pharmaceutical market works on the principle of reliance,” said an expert close to the WHO. A regulator in one country should be able to rely on the checks and assessments made by regulators in other WHO member states. If this doesn’t happen, the system starts to break down.
“This situation we have now is putting the system of reliance within the WHO under stress,” the source added.
The first Indian firm in the spotlight is Maiden Pharmaceuticals, which is based in the northern state of Haryana. It manufactured the syrup medicine that has been blamed for the deaths in Gambia.
Only last month two of its executives were sentenced to two-and-half years in jail for exporting substandard drugs to Vietnam a decade ago.
“This court has come to the conclusion that the complainant/prosecution has duly proved the charge ... beyond a shadow of reasonable doubt,” the judge, Sanjeev Arya, told the court.

A member of the Gambian Red Cross looks through sacks of seized cough syrups in Banjul - MILAN BERCKMANS/AFP via Getty Images)
Despite its track record, the Indian authorities allowed Maiden to continue to trade and to export products internationally, including to Gambia.
Officials only launched an investigation into the company after four of its medicines exported to Gambia (Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup) were found by the WHO to be contaminated.
India’s drug watchdog, the Central Drugs Standard Control Organisation (CDSCO), said it found multiple violations of good manufacturing practices and testing at Maiden but then claimed that the cough syrups themselves were clean.
This is despite independent laboratories in Switzerland, Ghana, and France all finding high levels of toxins in the syrups taken by the Gambian children.
“The Indian government has taken an appalling position of shielding the company rather than doing a transparent and thorough investigation of the international incident,” said Malini Asoila, of the All India Drug Action Network (AIDAN), a patients’ rights campaign group.
Despite India boasting of having become “the world’s pharmacy” due to the scale of its medicines exports, it is unclear if useful information will ever be recovered from Maiden.
When the Indian regulator visited the company in the wake of the Gambia deaths, inspectors found the premises were undergoing “renovation”.
Equipment used for manufacturing and testing cough syrups was alleged to have gone missing. And key information on batch numbers and manufacturing and expiry dates could not be found, according to a report seen by the Telegraph.
The inspectors wrote: “Complete plant is found under renovation. The firm failed to produce the log books of equipments and instruments regarding manufacturing and testing for the drugs in question.”

Police is seen at the gate of an office of Marion Biotech, a healthcare and pharmaceutical company and a part of the Emenox Group, whose cough syrup has been linked to the deaths of children in Uzbekistan, in Noida, India - Anushree Fadnavis/REUTERS
The second Indian company caught up in the scandal is Marion Biotech, which operates out of Uttar Pradesh, a state in the north. The WHO said two of its medicines, Ambronol syrup and DOK-1 Max syrup, were found to have unacceptable levels of EG and or DEG after the deaths in Uzbekistan.
Last month the firm told the government of Uttar Pradesh that it was being blamed for the deaths in Uzbekistan “to malign the image of India and the company”.
However, three Marion Biotech employees have since been arrested after Indian government testing found that 22 out of 36 cough syrup samples taken from the company contained EG and DEG.
This includes samples of drugs which were exported to Uzbekistan and consumed there, said Vaibhav Babbar, an inspector involved in the Marion probe.
“Because Marion’s drugs have gone to so many countries, I pray nothing happens elsewhere,” he told Reuters on Saturday. “The health ministry could issue an alert. They may do it. It will be good to issue an alert.”
A senior police officer involved in the arrests told local reporters: “These people were engaged in preparation and sale of fake drugs which caused serious harm to the public.
“Besides three suspects who have been arrested, there are two more directors of the company for whom searches are underway and they will be also arrested soon.”
The WHO is understood not to have received any information from the Indian authorities about the company despite asking for information.
“This has been going on for several months now and we are still struggling to find the contaminated material,” said the expert source.
Appealing for assistance from regulators globally, they added: “What we have is a spider’s web …. we have hundreds of deaths.”
While Marion Biotech and Maiden Pharmaceuticals have been linked to the deaths in Gambia and Uzbekistan, the fatalities in Indonesia have been tied to local companies.
The WHO and Indonesian authorities issued recall warnings in November for cough syrups made by four Indonesian manufacturers: PT Yarindo Farmatama; PT Universal Pharmaceutical; PT Konimex; and PT AFI Pharma.
The Indonesian companies involved have either denied that their products have been contaminated or declined to comment while investigations are ongoing.
But a lawyer for PT Universal Pharmaceutical Industries, Hermansyah Hutagalung, last month blamed the firm’s suppliers.
“Chase the suppliers, they’re the real criminals,” he said at the time. “They’re the ones that forge raw ingredients by falsifying raw ingredient documents all the way to pharmaceutical companies.”
‘Culprits must be punished’
The recent tragedies in Gambia, Uzbekistan and Indonesia are far from unique. Mass medicine poisonings involving DEG or EG contamination have a history going back nearly a century.
The first was in the United States in 1937 when DEG was used as a solvent in a liquid antibiotic, killing 100 people – a third of them children. The catastrophe ultimately led to the creation of U.S. Food and Drug Administration (FDA)
Yet in the last 30 years alone, there have been similar poisonings in Panama, China, Haiti, Bangladesh, Argentina, Nigeria, and India.
The problem is not typically caused by the active pharmaceutical molecules in medicines, say experts, but with the industrially-produced chemicals used to bulk up and stabilise the medicinal mixture, such as glycerine and propylene glycol.
These solvents can be contaminated, or even totally replaced, with DEG which has similar chemical properties. This can happen either through mislabeled products, human error or intentional adulteration.
Smita Salunke, chief scientific officer with the European Paediatric Formulation Initiative (EuPFI), which works to make children’s medicines better and safer, told the Telegraph that DEG-contaminated glycerine had been responsible for many previous poisoning incidents.

parents of children, who they alleged were either killed or left sickened by tainted cough syrups, attend a hearing as part of a lawsuit at the South Jakarta district court, while wearing black shirts reading "I thought it was medicine, it was poison" to the court - GEMMA CAHYA/AFP via Getty Images
“Similar chemical properties of glycerine, propylene glycol and DEG make it possible for DEG to be passed as counterfeit chemicals deliberately or sometimes accidently,” she said.
Naresh Goyal, director of Maiden Pharmaceuticals, told the Telegraph he thought suppliers should be investigated for possible contamination.
He said: “There might be some intermediate which has come and is used by the companies. This may be one reason for the contamination, possibly.
“We are getting a quantity of 20 or 50 drums of propylene glycol and we are not testing every drum. We are testing the batches. It is possible that a contaminated batch has come which has been used somewhere.”
India’s drug regulator is reported to be investigating whether propylene glycol and glycerine used in the manufacture of cough syrup at Maiden might have been the source of the EG or DEG contamination.
The WHO last month also advised cough syrup manufacturers to check contamination in a number of ingredients, including propylene glycol and glycerine.
“The key question is this: Is the contaminated product in the supply chain or is it a simple substitution or accident?” said the expert source close to the WHO.
Enforcing standards in India’s drug industry can prove especially challenging due to the shadowy nexus of corrupt drug officials and pharmaceutical companies that has emerged within the country.
“Across India, there are drug officials who help the pharma companies to push through the substandard or fake medicines. The government needs to increase surveillance and monitoring systems,” a retired senior government drug official told The Telegraph.
“Culprits must be punished in faster ways so that no one can think of compromising the quality of drugs,” said Dr GN Singh, a former drugs controller general. “There is laxity on part of the whole system.”
Maiden Pharmaceuticals, Marion Biotech, India’s Central Drugs Standard Control Organisation, and the Indian health ministry were all approached for comment but did not respond.
Protect yourself and your family by learning more about Global Health Security
No comments:
Post a Comment