New strategy for drug design: Keeping copper atoms closer to keep bacteria away
The discovery of antibiotics was a huge breakthrough in medicine, which helped save countless lives. Unfortunately, their widespread use has led to the rapid evolution of highly resistant bacterial strains, which threaten to take humanity back to square one in the fight against infectious diseases. Even though researchers are seeking new design concepts for antibacterial drugs, the overall development of new agents is currently on the decline.
To tackle this serious problem, scientists at Tokyo University of Science, Japan, are exploring a novel approach to boost the in vivo antibacterial activity of hydrogen peroxide (H2O2), a commonly used disinfectant. In a recent study published in Macromolecular Rapid Communications, a team led by Assistant Professor Shigehito Osawa and Professor Hidenori Otsuka reported their success in enhancing H2O2 activity using carefully tailored copper-containing polymers.
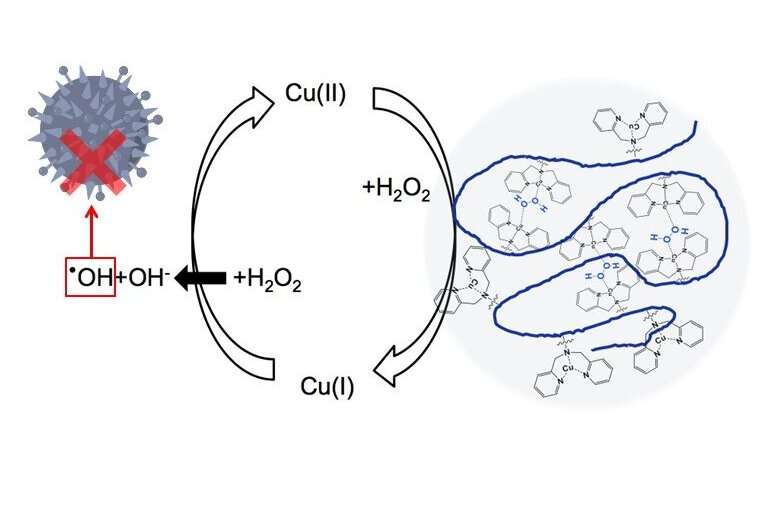
To understand their approach, it helps to know how H2O2 acts against bacteria in the first place, and the role that copper plays. H2O2 can be decomposed into a hydroxyl radical (OH) and a hydroxide anion (OH−), the former of which is highly toxic to bacteria as it readily destroys certain biomolecules. Copper in its first oxidation state, Cu(I), can catalyze the splitting of H2O2 into a hydroxyl radical and a hydroxide anion, turning into Cu(II) in the process through oxidation. Curiously, H2O2 can also catalyze the reduction of Cu(II) to Cu(I), but only if this reaction is somehow facilitated. One way to achieve this is to have Cu(II)-containing complexes get close enough together.
However, when using Cu(II)-containing complexes dissolved in a solution, the only way for them to come close together is by accidentally bumping into each other, which requires an excessively high concentration of copper. The team found a workaround to this issue by drawing inspiration from cellular chemistry, as Dr. Osawa explains: "In living organisms, copper forms complexes with proteins to efficiently catalyze redox reactions. For example, tyrosinase has two copper complex sites in close proximity to each other, which facilitates the formation of reaction intermediates between oxygen species and copper complexes. We thought we could leverage this type of mechanism in artificially produced polymers with copper complexes, even if dispersed in a solution."
With this idea, the researchers developed a long polymer chain with dipicolylamine (DPA) as copper-containing complexes. These DPA–copper complexes were attached to the long polymer backbone as "pendant groups." When these polymers are dispersed in a solution, the Cu(II) atoms in the pendant groups are kept in close proximity and locally high densities, vastly increasing the chances that two of them will be close enough to be reduced to Cu(I) by H2O2. Through various experiments, the scientists demonstrated that the use of these tailored polymers resulted in higher catalytic activity for the splitting of H2O2, resulting in more OH even for lower concentrations of copper. Further tests using Escherichia coli cultures showed that these polymers greatly enhanced the antibacterial potential of H2O2.
While the results of this study open up a new design avenue for antimicrobial drugs, there may also be useful applications in the food industry as well. "Because copper is an essential nutrient for living organisms, the antibacterial agent developed in this study holds promise as an efficient food preservative, which could contribute to increasing the variety of foods that can be preserved over long shelf times," highlights Dr. Osawa. Let us hope this new strategy makes it easier for us to keep microscopic menaces at bay.
No comments:
Post a Comment