Using an ethylene carbonate solvent with a sodium iodide salt to create a new kind of refrigerator
A pair of researchers at Lawrence Berkely National Laboratory used a commonly known, naturally occurring phenomenon to build a new kind of environmentally safe refrigerator.
In their paper published in the journal Science, Drew Lilley and Ravi Prasher describe how expanding on the idea of using salt to melt road ice to design and build a new kind of refrigerator. Emmanuel Defay, with the Luxembourg Institute of Science and Technology, has published a Perspective piece in the same journal issue outlining the work done by the pair in California.
For many years, people around the world have used salt to melt road ice to make travel easier. Though technically, the salt does not melt the ice, its dark color attracts heat, allowing the ice below it to melt, which than allows the salt to mix with the water. And it does not refreeze because the salt dramatically lowers the freezing point of the water.
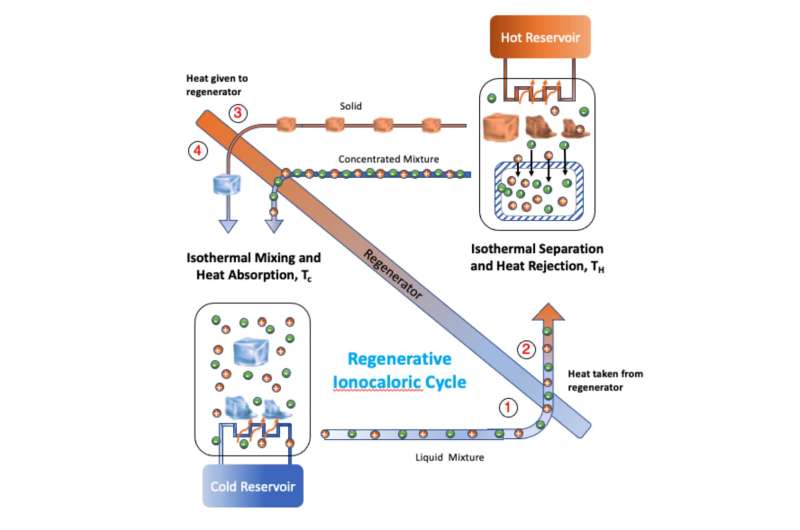
It was on this part of the process that the researchers focused. They noted that a similar process could result in cooling a material simply by mixing it with sodium iodide (NaI) salt due to the phase transition. The second material in this case was an ethylene carbonate (EC) solvent. They further noted that repeatedly cooling a material should also cool the environment in which it is contained. And to make that happen, all they had to do was remove the salt, and then add it again.
The researchers call their process "ionocaloric" refrigeration, and built such a refrigerator to prove that it was viable. They started with a box and then added a mixing device to mix their two ingredients and another device that performed electrodialysis to remove the salt. Then tested the resulting device to determine if it would keep the temperature inside the box at a steady cool temperature, and if so, if it was more or less efficient than other refrigeration devices.
Their testing showed that their refrigerator was able to maintain a cool temperature and that it was approximately as efficient as refrigerators now on the market. The big advantage of the approach is that it does not emit any hydrofluorocarbons or other pollutants. They acknowledge that it does have one drawback—it takes quite a while for the mixed solution to cool.
Emmanuel Defay, Cool it, with a pinch of salt, Science (2022). DOI: 10.1126/science.adf5114
Journal information: Science
© 2022 Science X Network
No comments:
Post a Comment