FEBRUARY 24, 2024
Pharmaceutical companies last year launched new US drugs at prices 35 per cent higher than in 2022, reflecting in part the industry's embrace of expensive therapies for rare diseases like muscular dystrophy, a Reuters analysis found.
The median annual list price for a new drug was US$300,000 (S$402,900) in 2023, according to the Reuters analysis of 47 medicines, up from US$222,000 a year earlier. In 2021, the median annual price was US$180,000, opens new tab for the 30 drugs first marketed through mid-July, according to a study published in JAMA.
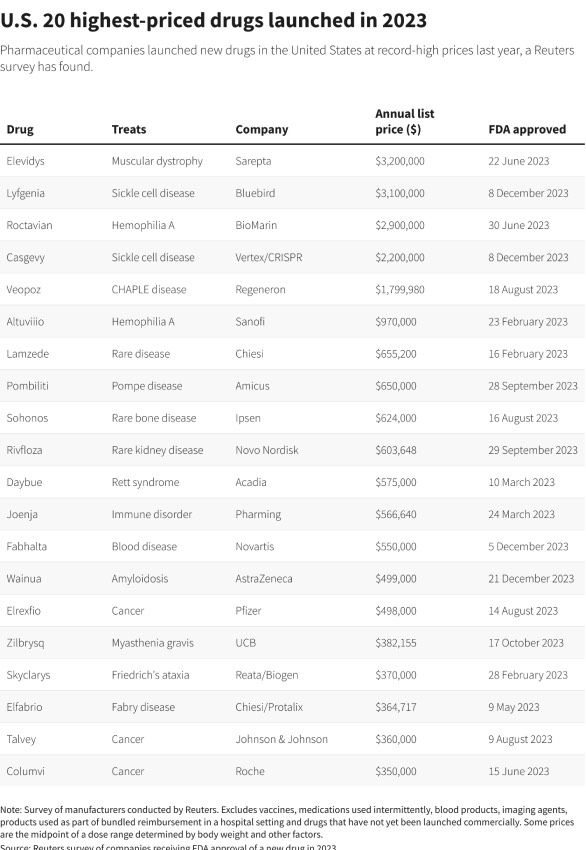
PHOTO: Reuters graphics
More than half of the new products approved by the Food and Drug Administration in 2023 and 2022 were for orphan diseases, meaning they affect fewer than 200,000 Americans, and some are not expected to become big sellers. The orphan rate is slightly higher than the 49 per cent seen in the previous five years, opens new tab.
A high price is justified for a drug with a lot of value to patients, but "prices are just going up and up without any clear rationale as to why," said Dan Ollendorf, chief scientific officer at the Institute for Clinical and Economic Review (ICER), an influential group that evaluates the value and prices of medicines.
He said many rare disease and cancer drugs are not being priced relative to their benefit, but the lack of alternatives gives manufacturers negotiating leverage.
Reuters analysed 55 novel drugs approved by the FDA last year, up from 37 in 2022. The agency's biologic division approved 17 new products, including four gene therapies.
The analysis excludes vaccines and drugs used intermittently such as Pfizer's, opens new tab Covid-19 antiviral Paxlovid. It also excludes drugs that have not yet launched commercially.
Of the 47 medications included in the analysis, the highest price for drugs taken consistently was US$1.8 million a year for Regeneron's, opens new tab Veopoz for CHAPLE disease, an inherited condition diagnosed in fewer than 100 people worldwide in which parts of the immune system become overactive.
The lowest annual price was US$576 for diabetes drug Brenzavvy, sold by TheracosBio in partnership with Mark Cuban's Cost Plus Drugs online pharmacy.
ICER's Ollendorf said it is too early to tell whether TheracosBio will succeed with its strategy to "blow up" the typical drug contracting model by selling a medication in a widely-used class at a much lower price than the competition.
TheracosBio CEO Brian Connelly said Brenzavvy "sales are increasing at a great clip," but declined to provide details.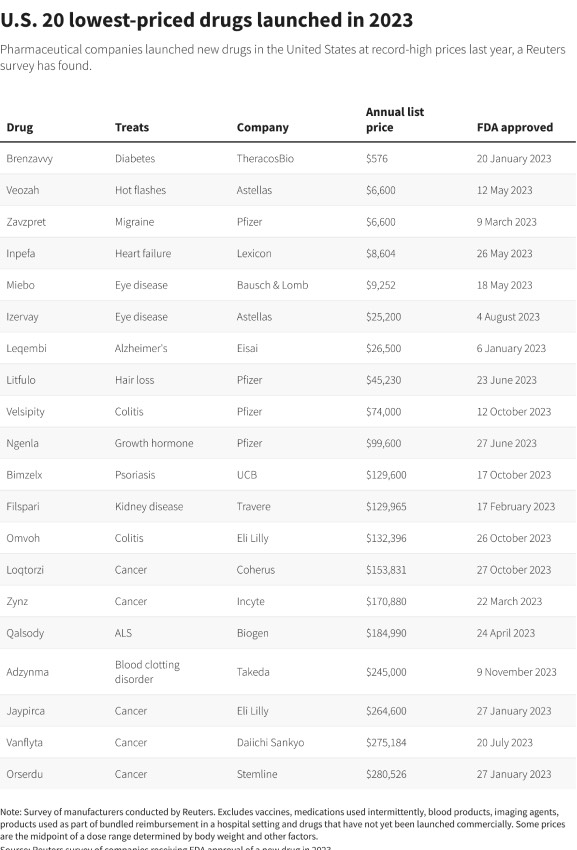
More than half of the new products approved by the Food and Drug Administration in 2023 and 2022 were for orphan diseases, meaning they affect fewer than 200,000 Americans, and some are not expected to become big sellers. The orphan rate is slightly higher than the 49 per cent seen in the previous five years, opens new tab.
A high price is justified for a drug with a lot of value to patients, but "prices are just going up and up without any clear rationale as to why," said Dan Ollendorf, chief scientific officer at the Institute for Clinical and Economic Review (ICER), an influential group that evaluates the value and prices of medicines.
He said many rare disease and cancer drugs are not being priced relative to their benefit, but the lack of alternatives gives manufacturers negotiating leverage.
Reuters analysed 55 novel drugs approved by the FDA last year, up from 37 in 2022. The agency's biologic division approved 17 new products, including four gene therapies.
The analysis excludes vaccines and drugs used intermittently such as Pfizer's, opens new tab Covid-19 antiviral Paxlovid. It also excludes drugs that have not yet launched commercially.
Of the 47 medications included in the analysis, the highest price for drugs taken consistently was US$1.8 million a year for Regeneron's, opens new tab Veopoz for CHAPLE disease, an inherited condition diagnosed in fewer than 100 people worldwide in which parts of the immune system become overactive.
The lowest annual price was US$576 for diabetes drug Brenzavvy, sold by TheracosBio in partnership with Mark Cuban's Cost Plus Drugs online pharmacy.
ICER's Ollendorf said it is too early to tell whether TheracosBio will succeed with its strategy to "blow up" the typical drug contracting model by selling a medication in a widely-used class at a much lower price than the competition.
TheracosBio CEO Brian Connelly said Brenzavvy "sales are increasing at a great clip," but declined to provide details.

PHOTO: Reuters graphics
Market incentives
Gene therapies, which are one-time treatments, range in price from US$2.2 million for sickle cell disease treatment Casgevy from Vertex, opens new tab and CRISPR, opens new tab, to US$3.2 million for Sarepta's, opens new tab muscular dystrophy therapy Elevydis.
The US gives drug manufacturers market exclusivity, fee waivers, direct funding for research and development, and tax credits for such orphan treatments.
"We created a lot of incentives for innovation for rare diseases and the market responded," said Dana Goldman, director of the USC Schaeffer Center for Health Policy & Economics. "The hope is that eventually we will see some therapeutic competition in this space and bring prices down."
The 2022 Inflation Reduction Act limits how much drugmakers can raise prices for treatments offered under Medicare, the federal health plan for people age 65 and over. The legislation does not limit what can be charged for new medicines.
"It means you are encouraging companies to launch at high prices," Goldman said.
Drugmakers stress they do not determine what US patients end up paying. Many offer savings cards and other programs to reduce out-of-pocket costs, while health insurers can receive discounts and rebates from manufacturer list prices, especially if competing treatments are available.
Drugmakers also say new medicines offer cost-saving value, including the possibility of fewer emergency room visits and hospital stays.
Boston Consulting Group forecast that 24 per cent of 2023's new drugs will reach blockbuster status - annual sales of over US$1 billion - versus 35 per cent from 2022's crop.
As patents expire, lower-cost generics mitigate prescription drug price inflation, which in the 12 months through December was roughly in line with broader inflation at 3.3 per cent, according to US government data.
No comments:
Post a Comment